 |
PDBsum entry 5a5e
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of murd ligase from escherichia coli
|
|
Structure:
|
 |
Udp-n-acetylmuramoylalanine--d-glutamate ligase. Chain: a. Synonym: d-glutamic acid-adding enzyme, udp-n-acetylmuramoyl-l-alany l-d-glutamate synthetase, murd ligase. Engineered: yes
|
|
Source:
|
 |
Escherichia coli k-12. Organism_taxid: 83333. Expressed in: escherichia coli dh5[alpha]. Expression_system_taxid: 668369
|
|
Resolution:
|
 |
|
1.84Å
|
R-factor:
|
0.191
|
R-free:
|
0.231
|
|
|
Authors:
|
 |
R.Sink,M.Kotnik,A.Zega,H.Barreteau,S.Gobec,D.Blanot,A.Dessen, C.Contreras-Martel
|
|
Key ref:
|
 |
R.Šink
et al.
(2016).
Crystallographic Study of Peptidoglycan Biosynthesis Enzyme MurD: Domain Movement Revisited.
Plos One,
11,
e0152075.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
17-Jun-15
|
Release date:
|
13-Apr-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P14900
(MURD_ECOLI) -
UDP-N-acetylmuramoylalanine--D-glutamate ligase from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
438 a.a.
431 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.2.9
- UDP-N-acetylmuramoyl-L-alanine--D-glutamate ligase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Peptidoglycan Biosynthesis (Part 1)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
UDP-N-acetyl-alpha-D-muramoyl-L-alanine + D-glutamate + ATP = UDP-N- acetyl-alpha-D-muramoyl-L-alanyl-D-glutamate + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
UDP-N-acetyl-alpha-D-muramoyl-L-alanine
|
+
|
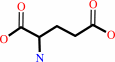 D-glutamate
D-glutamate
|
+
|
 ATP
ATP
|
=
|
UDP-N- acetyl-alpha-D-muramoyl-L-alanyl-D-glutamate
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Plos One
11:e0152075
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystallographic Study of Peptidoglycan Biosynthesis Enzyme MurD: Domain Movement Revisited.
|
|
R.Šink,
M.Kotnik,
A.Zega,
H.Barreteau,
S.Gobec,
D.Blanot,
A.Dessen,
C.Contreras-Martel.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The biosynthetic pathway of peptidoglycan, an essential component of bacterial
cell wall, is a well-recognized target for antibiotic development. Peptidoglycan
precursors are synthesized in the bacterial cytosol by various enzymes including
the ATP-hydrolyzing Mur ligases, which catalyze the stepwise addition of amino
acids to a UDP-MurNAc precursor to yield UDP-MurNAc-pentapeptide. MurD catalyzes
the addition of D-glutamic acid to UDP-MurNAc-L-Ala in the presence of ATP;
structural and biochemical studies have suggested the binding of the substrates
with an ordered kinetic mechanism in which ligand binding inevitably closes the
active site. In this work, we challenge this assumption by reporting the crystal
structures of intermediate forms of MurD either in the absence of ligands or in
the presence of small molecules. A detailed analysis provides insight into the
events that lead to the closure of MurD and reveals that minor structural
modifications contribute to major overall conformation alterations. These novel
insights will be instrumental in the development of new potential antibiotics
designed to target the peptidoglycan biosynthetic pathway.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

