 |
PDBsum entry 4zwx

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Lyase/lyase inhibitor
|
PDB id
|
|

|

|
4zwx
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.4.2.1.1
- carbonic anhydrase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydrogencarbonate + H+ = CO2 + H2O
|
 |
 |
 |
 |
 |
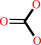 hydrogencarbonate
hydrogencarbonate
|
+
|
H(+)
|
=
|
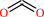 CO2
CO2
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Zn(2+)
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.4.2.1.69
- cyanamide hydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
urea = cyanamide + H2O
|
 |
 |
 |
 |
 |
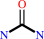 urea
urea
|
=
|
 cyanamide
cyanamide
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
58:6630-6638
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
Mapping Selective Inhibition of the Cancer-Related Carbonic Anhydrase IX Using Structure-Activity Relationships of Glucosyl-Based Sulfamates.
|
|
B.P.Mahon,
C.L.Lomelino,
J.Ladwig,
G.M.Rankin,
J.M.Driscoll,
A.L.Salguero,
M.A.Pinard,
D.Vullo,
C.T.Supuran,
S.A.Poulsen,
R.McKenna.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Inhibition of human carbonic anhydrase IX (hCA IX) has shown to be
therapeutically advantageous for treating many types of highly aggressive
cancers. However, designing selective inhibitors for hCA IX has been difficult
due to its high structural homology and sequence similarity with off-target
hCAs. Recently, the use of glucosyl sulfamate inhibitors has shown promise as
selective inhibitors for hCA IX. In this study, we present five X-ray crystal
structures, determined to a resolution of 1.7 Å or better, of both hCA II (a
ubiquitous CA) and an engineered hCA IX-mimic in complex with selected glucosyl
sulfamates and structurally rationalize mechanisms for hCA IX selectivity.
Results from this study have allowed us, for the first time, to empirically
"map" key interactions of the hCA IX active site in order to establish
parameters needed to design novel hCA IX selective inhibitors.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

