 |
PDBsum entry 4zqi
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of apo d-alanine-d-alanine ligase(ddl) from yersinia pestis
|
|
Structure:
|
 |
D-alanine--d-alanine ligase. Chain: a, b, c, d. Synonym: d-ala-d-ala ligase,d-alanylalanine synthetase. Engineered: yes
|
|
Source:
|
 |
Yersinia pestis. Organism_taxid: 632. Gene: ddl, ddlb. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.30Å
|
R-factor:
|
0.206
|
R-free:
|
0.248
|
|
|
Authors:
|
 |
H.-T.Tran,L.-W.Kang,M.-K.Hong,H.P.T.Ngo,K.H.Huynh,Y.J.Ahn
|
|
Key ref:
|
 |
H.T.Tran
et al.
(2016).
Structure of D-alanine-D-alanine ligase from Yersinia pestis: nucleotide phosphate recognition by the serine loop.
Acta Crystallogr D Struct Biol,
72,
12-21.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
10-May-15
|
Release date:
|
13-Jan-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q8ZIE7
(DDL_YERPE) -
D-alanine--D-alanine ligase from Yersinia pestis
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
306 a.a.
292 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.2.4
- D-alanine--D-alanine ligase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Peptidoglycan Biosynthesis (Part 1)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 D-alanine + ATP = D-alanyl-D-alanine + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
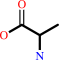 2
×
D-alanine
2
×
D-alanine
|
+
|
 ATP
ATP
|
=
|
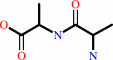 D-alanyl-D-alanine
D-alanyl-D-alanine
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Struct Biol
72:12-21
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of D-alanine-D-alanine ligase from Yersinia pestis: nucleotide phosphate recognition by the serine loop.
|
|
H.T.Tran,
M.K.Hong,
H.P.Ngo,
K.H.Huynh,
Y.J.Ahn,
Z.Wang,
L.W.Kang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
D-Alanyl-D-alanine is an essential precursor of bacterial peptidoglycan and is
synthesized by D-alanine-D-alanine ligase (DDL) with hydrolysis of ATP; this
reaction makes DDL an important drug target for the development of antibacterial
agents. Five crystal structures of DDL from Yersinia pestis (YpDDL) were
determined at 1.7-2.5 Å resolution: apo, AMP-bound, ADP-bound, adenosine
5'-(β,γ-imido)triphosphate-bound, and D-alanyl-D-alanine- and ADP-bound
structures. YpDDL consists of three domains, in which four loops, loop 1, loop 2
(the serine loop), loop 3 (the ω-loop) and loop 4, constitute the binding sites
for two D-alanine molecules and one ATP molecule. Some of them, especially the
serine loop and the ω-loop, show flexible conformations, and the serine loop is
mainly responsible for the conformational change in substrate nucleotide
phosphates. Enzyme-kinetics assays were carried out for both the D-alanine and
ATP substrates and a substrate-binding mechanism was proposed for YpDDL
involving conformational changes of the loops.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

