 |
PDBsum entry 4zab
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.1.1.102
- phenacrylate decarboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
(E)-4-coumarate + H+ = 4-vinylphenol + CO2
|
|
2.
|
(E)-cinnamate + H+ = styrene + CO2
|
|
3.
|
(E)-ferulate + H+ = 2-methoxy-4-vinylphenol + CO2
|
|
 |
 |
 |
 |
 |
(E)-4-coumarate
|
+
|
H(+)
|
=
|
4-vinylphenol
Bound ligand (Het Group name = )
matches with 75.00% similarity
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
(E)-cinnamate
Bound ligand (Het Group name = )
matches with 91.67% similarity
|
+
|
H(+)
|
=
|
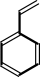 styrene
styrene
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
(E)-ferulate
|
+
|
H(+)
|
=
|
2-methoxy-4-vinylphenol
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Prenyl-FMNH(2)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Nature
522:497-501
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
New cofactor supports α,β-unsaturated acid decarboxylation via 1,3-dipolar cycloaddition.
|
|
K.A.Payne,
M.D.White,
K.Fisher,
B.Khara,
S.S.Bailey,
D.Parker,
N.J.Rattray,
D.K.Trivedi,
R.Goodacre,
R.Beveridge,
P.Barran,
S.E.Rigby,
N.S.Scrutton,
S.Hay,
D.Leys.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The bacterial ubiD and ubiX or the homologous fungal fdc1 and pad1 genes have
been implicated in the non-oxidative reversible decarboxylation of aromatic
substrates, and play a pivotal role in bacterial ubiquinone (also known as
coenzyme Q) biosynthesis or microbial biodegradation of aromatic compounds,
respectively. Despite biochemical studies on individual gene products, the
composition and cofactor requirement of the enzyme responsible for in vivo
decarboxylase activity remained unclear. Here we show that Fdc1 is solely
responsible for the reversible decarboxylase activity, and that it requires a
new type of cofactor: a prenylated flavin synthesized by the associated
UbiX/Pad1. Atomic resolution crystal structures reveal that two distinct isomers
of the oxidized cofactor can be observed, an isoalloxazine N5-iminium adduct and
a N5 secondary ketimine species with markedly altered ring structure, both
having azomethine ylide character. Substrate binding positions the dipolarophile
enoic acid group directly above the azomethine ylide group. The structure of a
covalent inhibitor-cofactor adduct suggests that 1,3-dipolar cycloaddition
chemistry supports reversible decarboxylation in these enzymes. Although
1,3-dipolar cycloaddition is commonly used in organic chemistry, we propose that
this presents the first example, to our knowledge, of an enzymatic 1,3-dipolar
cycloaddition reaction. Our model for Fdc1/UbiD catalysis offers new routes in
alkene hydrocarbon production or aryl (de)carboxylation.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

