 |
PDBsum entry 4z0l

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase/oxidoreductase inhibitor
|
PDB id
|
|

|

|
4z0l
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase/oxidoreductase inhibitor
|
 |
|
Title:
|
 |
The murine cyclooxygenase-2 complexed with a nido-dicarbaborate- containing indomethacin derivative
|
|
Structure:
|
 |
Prostaglandin g/h synthase 2. Chain: a, b, c, d. Fragment: unp residues 18-604. Synonym: cyclooxygenase-2,cox-2,glucocorticoid-regulated inflammatory cyclooxygenase,gripghs,macrophage activation-associated marker protein p71/73,pes-2,phs ii,prostaglandin h2 synthase 2,pghs-2, prostaglandin-endoperoxide synthase 2,tis10 protein. Engineered: yes
|
|
Source:
|
 |
Mus musculus. Mouse. Organism_taxid: 10090. Gene: ptgs2, cox-2, cox2, pghs-b, tis10. Expressed in: spodoptera frugiperda. Expression_system_taxid: 7108. Expression_system_cell_line: sf21.
|
|
Resolution:
|
 |
|
2.29Å
|
R-factor:
|
0.212
|
R-free:
|
0.250
|
|
|
Authors:
|
 |
S.Xu,W.Neumann,S.Banerjee,E.Hey-Hawkins,L.J.Marnett
|
|
Key ref:
|
 |
W.Neumann
et al.
(2016).
nido-Dicarbaborate Induces Potent and Selective Inhibition of Cyclooxygenase-2.
Chemmedchem,
11,
175-178.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
26-Mar-15
|
Release date:
|
10-Jun-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q05769
(PGH2_MOUSE) -
Prostaglandin G/H synthase 2 from Mus musculus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
604 a.a.
551 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.99.1
- prostaglandin-endoperoxide synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate + AH2 + 2 O2 = prostaglandin H2 + A + H2O
|
 |
 |
 |
 |
 |
(5Z,8Z,11Z,14Z)-eicosatetraenoate
|
+
|
AH2
|
+
|
 2
×
O2
2
×
O2
|
=
|
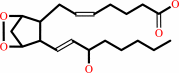 prostaglandin H2
prostaglandin H2
|
+
|
|
+
|
H2O
Bound ligand (Het Group name = )
matches with 51.11% similarity
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Chemmedchem
11:175-178
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
nido-Dicarbaborate Induces Potent and Selective Inhibition of Cyclooxygenase-2.
|
|
W.Neumann,
S.Xu,
M.B.Sárosi,
M.S.Scholz,
B.C.Crews,
K.Ghebreselasie,
S.Banerjee,
L.J.Marnett,
E.Hey-Hawkins.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Carbaboranes are increasingly studied as pharmacophores, particularly as
replacements for aromatic systems. However, especially ortho-carbaborane is
prone to degradation of the cluster, which hampers biological application. This
study demonstrates that deboronation of the cluster may not only lead to a more
active analogue, but can also improve the solubility and stability of a
carbaborane-containing inhibitor. Notably, introduction of a nido-dicarbaborate
cluster into the cyclooxygenase (COX) inhibitor indomethacin results in
remarkably increased inhibitory potency and selectivity for COX-2 relative to
the respective phenyl analogue. The first crystal structure of a
carbaborane-containing inhibitor bound to COX-2 further reveals a novel binding
mode for the inhibitor that is strikingly different from that of indomethacin.
These results indicate that nido-dicarbaborate is a promising pharmacophore that
exhibits properties which are also highly beneficial for its introduction into
other inhibitor classes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

