 |
PDBsum entry 4pvs
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of fully-cleaved human l-asparaginase protein in complex with l-aspartate
|
|
Structure:
|
 |
Isoaspartyl peptidase/l-asparaginase. Chain: a, b. Synonym: asparaginase-like protein 1, beta-aspartyl-peptidase, isoaspartyl dipeptidase, l-asparagine amidohydrolase, isoaspartyl peptidase/l-asparaginase alpha chain, isoaspartyl peptidase/l- asparaginase beta chain. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: alp, asrgl1, crash. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
1.84Å
|
R-factor:
|
0.171
|
R-free:
|
0.216
|
|
|
Authors:
|
 |
J.Nomme,A.Lavie
|
|
Key ref:
|
 |
J.Nomme
et al.
(2012).
Structures of apo and product-bound human L-asparaginase: insights into the mechanism of autoproteolysis and substrate hydrolysis.
Biochemistry,
51,
6816-6826.
PubMed id:
|
 |
|
Date:
|
 |
|
18-Mar-14
|
Release date:
|
26-Mar-14
|
|
|
Supersedes:
|

|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q7L266
(ASGL1_HUMAN) -
Isoaspartyl peptidase/L-asparaginase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
308 a.a.
295 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.3.4.19.5
- beta-aspartyl-peptidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Cleavage of a beta-linked aspartic residue from the N-terminus of a polypeptide.
|
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.5.1.1
- asparaginase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
L-asparagine + H2O = L-aspartate + NH4+
|
 |
 |
 |
 |
 |
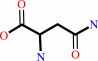 L-asparagine
L-asparagine
|
+
|
H2O
|
=
|
L-aspartate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
51:6816-6826
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structures of apo and product-bound human L-asparaginase: insights into the mechanism of autoproteolysis and substrate hydrolysis.
|
|
J.Nomme,
Y.Su,
M.Konrad,
A.Lavie.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

