 |
PDBsum entry 4n4l

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
4n4l
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.7.2.9
- hydroxylamine oxidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
hydroxylamine + 3 Fe(III)-[cytochrome c] = nitric oxide + 3 Fe(II)- [cytochrome c] + 3 H+
|
 |
 |
 |
 |
 |
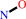 hydroxylamine
hydroxylamine
|
+
|
3
×
Fe(III)-[cytochrome c]
|
=
|
 nitric oxide
nitric oxide
|
+
|
3
×
Fe(II)- [cytochrome c]
|
+
|
3
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
289:1228-1242
(2014)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis of biological NO generation by octaheme oxidoreductases.
|
|
W.J.Maalcke,
A.Dietl,
S.J.Marritt,
J.N.Butt,
M.S.Jetten,
J.T.Keltjens,
T.R.Barends,
B.Kartal.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Nitric oxide is an important molecule in all domains of life with significant
biological functions in both pro- and eukaryotes. Anaerobic ammonium-oxidizing
(anammox) bacteria that contribute substantially to the release of fixed
nitrogen into the atmosphere use the oxidizing power of NO to activate inert
ammonium into hydrazine (N2H4). Here, we describe an enzyme from the anammox
bacterium Kuenenia stuttgartiensis that uses a novel pathway to make NO from
hydroxylamine. This new enzyme is related to octaheme hydroxylamine
oxidoreductase, a key protein in aerobic ammonium-oxidizing bacteria. By a
multiphasic approach including the determination of the crystal structure of the
K. stuttgartiensis enzyme at 1.8 Å resolution and refinement and reassessment
of the hydroxylamine oxidoreductase structure from Nitrosomonas europaea, both
in the presence and absence of their substrates, we propose a model for NO
formation by the K. stuttgartiensis enzyme. Our results expand the understanding
of the functions that the widespread family of octaheme proteins have.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

