 |
PDBsum entry 4mcd

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/transferase inhibitor
|
PDB id
|
|

|

|
4mcd
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.1.228
- tRNA (guanine(37)-N(1))-methyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
guanosine37 in tRNA + S-adenosyl-L-methionine = N1- methylguanosine37 in tRNA + S-adenosyl-L-homocysteine + H+
|
 |
 |
 |
 |
 |
guanosine(37) in tRNA
|
+
|
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
N(1)- methylguanosine(37) in tRNA
|
+
|
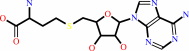 S-adenosyl-L-homocysteine
S-adenosyl-L-homocysteine
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
56:7278-7288
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
Selective inhibitors of bacterial t-RNA-(N(1)G37) methyltransferase (TrmD) that demonstrate novel ordering of the lid domain.
|
|
P.J.Hill,
A.Abibi,
R.Albert,
B.Andrews,
M.M.Gagnon,
N.Gao,
T.Grebe,
L.I.Hajec,
J.Huang,
S.Livchak,
S.D.Lahiri,
D.C.McKinney,
J.Thresher,
H.Wang,
N.Olivier,
E.T.Buurman.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The tRNA-(N(1)G37) methyltransferase (TrmD) is essential for growth and highly
conserved in both Gram-positive and Gram-negative bacterial pathogens.
Additionally, TrmD is very distinct from its human orthologue TRM5 and thus is a
suitable target for the design of novel antibacterials. Screening of a
collection of compound fragments using Haemophilus influenzae TrmD identified
inhibitory, fused thieno-pyrimidones that were competitive with
S-adenosylmethionine (SAM), the physiological methyl donor substrate. Guided by
X-ray cocrystal structures, fragment 1 was elaborated into a nanomolar inhibitor
of a broad range of Gram-negative TrmD isozymes. These compounds demonstrated no
activity against representative human SAM utilizing enzymes, PRMT1 and SET7/9.
This is the first report of selective, nanomolar inhibitors of TrmD with
demonstrated ability to order the TrmD lid in the absence of tRNA.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

