 |
PDBsum entry 4jht

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase/oxidoreductase inhibitor
|
PDB id
|
|

|

|
4jht
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.11.33
- Dna oxidative demethylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a methylated nucleobase within DNA + 2-oxoglutarate + O2 = a nucleobase within DNA + formaldehyde + succinate + CO2
|
 |
 |
 |
 |
 |
methylated nucleobase within DNA
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
+
|
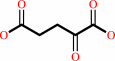 2-oxoglutarate
2-oxoglutarate
|
+
|
 O2
O2
|
=
|
nucleobase within DNA
|
+
|
formaldehyde
Bound ligand (Het Group name = )
matches with 75.00% similarity
|
+
|
 succinate
succinate
|
+
|
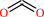 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Chem Sci
4:3110-3117
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
5-Carboxy-8-hydroxyquinoline is a Broad Spectrum 2-Oxoglutarate Oxygenase Inhibitor which Causes Iron Translocation.
|
|
R.J.Hopkinson,
A.Tumber,
C.Yapp,
R.Chowdhury,
W.Aik,
K.H.Che,
X.S.Li,
J.B.L.Kristensen,
O.N.F.King,
M.C.Chan,
K.K.Yeoh,
H.Choi,
L.J.Walport,
C.C.Thinnes,
J.T.Bush,
C.Lejeune,
A.M.Rydzik,
N.R.Rose,
E.A.Bagg,
M.A.McDonough,
T.Krojer,
W.W.Yue,
S.S.Ng,
L.Olsen,
P.E.Brennan,
U.Oppermann,
S.Muller-Knapp,
R.J.Klose,
P.J.Ratcliffe,
C.J.Schofield,
A.Kawamura.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
2-Oxoglutarate and iron dependent oxygenases are therapeutic targets for human
diseases. Using a representative 2OG oxygenase panel, we compare the inhibitory
activities of 5-carboxy-8-hydroxyquinoline (IOX1) and
4-carboxy-8-hydroxyquinoline (4C8HQ) with that of two other commonly used 2OG
oxygenase inhibitors,N-oxalylglycine (NOG) and 2,4-pyridinedicarboxylic
acid (2,4-PDCA). The results reveal that IOX1 has a broad spectrum of activity,
as demonstrated by the inhibition of transcription factor hydroxylases,
representatives of all 2OG dependent histone demethylase subfamilies, nucleic
acid demethylases and γ-butyrobetaine hydroxylase. Cellular assays show that,
unlike NOG and 2,4-PDCA, IOX1 is active against both cytosolic and nuclear 2OG
oxygenases without ester derivatisation. Unexpectedly, crystallographic studies
on these oxygenases demonstrate that IOX1, but not 4C8HQ, can cause
translocation of the active site metal, revealing a rare example of protein
ligand-induced metal movement.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

