 |
PDBsum entry 4ixh

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
4ixh
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Crystal structure of the catalytic domain of the inosine monophosphate dehydrogenase from cryptosporidium parvum
|
|
Structure:
|
 |
Inosine-5'-monophosphate dehydrogenase. Chain: a, b, c, d. Synonym: imp dehydrogenase, impd, impdh. Engineered: yes
|
|
Source:
|
 |
Cryptosporidium parvum. Organism_taxid: 5807. Gene: 56k.02, cgd6_20. Expressed in: escherichia coli. Expression_system_taxid: 511693.
|
|
Resolution:
|
 |
|
2.11Å
|
R-factor:
|
0.164
|
R-free:
|
0.210
|
|
|
Authors:
|
 |
Y.Kim,M.Makowska-Grzyska,M.Gu,M.Kavitha,L.Hedstrom,W.F.Anderson, A.Joachimiak,Center For Structural Genomics Of Infectious Diseases (Csgid)
|
|
Key ref:
|
 |
S.K.Gorla
et al.
(2013).
Optimization of benzoxazole-based inhibitors of Cryptosporidium parvum inosine 5'-monophosphate dehydrogenase.
J Med Chem,
56,
4028-4043.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
25-Jan-13
|
Release date:
|
03-Apr-13
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q8T6T2
(IMDH_CRYPV) -
Inosine-5'-monophosphate dehydrogenase from Cryptosporidium parvum
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
400 a.a.
336 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.1.1.205
- Imp dehydrogenase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
AMP and GMP Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
IMP + NAD+ + H2O = XMP + NADH + H+
|
 |
 |
 |
 |
 |
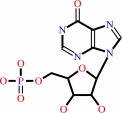 IMP
IMP
|
+
|
NAD(+)
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
H2O
|
=
|
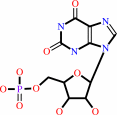 XMP
XMP
|
+
|
 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
56:4028-4043
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
Optimization of benzoxazole-based inhibitors of Cryptosporidium parvum inosine 5'-monophosphate dehydrogenase.
|
|
S.K.Gorla,
M.Kavitha,
M.Zhang,
J.E.Chin,
X.Liu,
B.Striepen,
M.Makowska-Grzyska,
Y.Kim,
A.Joachimiak,
L.Hedstrom,
G.D.Cuny.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cryptosporidium parvum is an enteric protozoan parasite that has emerged as a
major cause of diarrhea, malnutrition, and gastroenteritis and poses a potential
bioterrorism threat. C. parvum synthesizes guanine nucleotides from host
adenosine in a streamlined pathway that relies on inosine 5'-monophosphate
dehydrogenase (IMPDH). We have previously identified several parasite-selective
C. parvum IMPDH (CpIMPDH) inhibitors by high-throughput screening. In this
paper, we report the structure-activity relationship (SAR) for a series of
benzoxazole derivatives with many compounds demonstrating CpIMPDH IC50 values in
the nanomolar range and >500-fold selectivity over human IMPDH (hIMPDH).
Unlike previously reported CpIMPDH inhibitors, these compounds are competitive
inhibitors versus NAD(+). The SAR study reveals that pyridine and other small
heteroaromatic substituents are required at the 2-position of the benzoxazole
for potent inhibitory activity. In addition, several other SAR conclusions are
highlighted with regard to the benzoxazole and the amide portion of the
inhibitor, including preferred stereochemistry. An X-ray crystal structure of a
representative E·IMP·inhibitor complex is also presented. Overall, the
secondary amine derivative 15a demonstrated excellent CpIMPDH inhibitory
activity (IC50 = 0.5 ± 0.1 nM) and moderate stability (t1/2 = 44 min) in mouse
liver microsomes. Compound 73, the racemic version of 15a, also displayed superb
antiparasitic activity in a Toxoplasma gondii strain that relies on CpIMPDH
(EC50 = 20 ± 20 nM), and selectivity versus a wild-type T. gondii strain
(200-fold). No toxicity was observed (LD50 > 50 μM) against a panel of four
mammalian cells lines.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

