 |
PDBsum entry 4hs9
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.3
- triacylglycerol lipase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a triacylglycerol + H2O = a diacylglycerol + a fatty acid + H+
|
 |
 |
 |
 |
 |
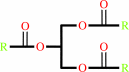 triacylglycerol
triacylglycerol
|
+
|
H2O
|
=
|
diacylglycerol
Bound ligand (Het Group name = )
matches with 50.00% similarity
|
+
|
 fatty acid
fatty acid
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Biotechnol Biofuels
6:70
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
Dieselzymes: development of a stable and methanol tolerant lipase for biodiesel production by directed evolution.
|
|
T.P.Korman,
B.Sahachartsiri,
D.M.Charbonneau,
G.L.Huang,
M.Beauregard,
J.U.Bowie.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
BACKGROUND: Biodiesels are methyl esters of fatty acids that are usually
produced by base catalyzed transesterification of triacylglyerol with methanol.
Some lipase enzymes are effective catalysts for biodiesel synthesis and have
many potential advantages over traditional base or acid catalyzed
trasesterification. Natural lipases are often rapidly inactivated by the high
methanol concentrations used for biodiesel synthesis, however, limiting their
practical use. The lipase from Proteus mirabilis is a particularly promising
catalyst for biodiesel synthesis as it produces high yields of methyl esters
even in the presence of large amounts of water and expresses very well in
Escherichia coli. However, since the Proteus mirabilis lipase is only moderately
stable and methanol tolerant, these properties need to be improved before the
enzyme can be used industrially. RESULTS: We employed directed evolution,
resulting in a Proteus mirabilis lipase variant with 13 mutations, which we call
Dieselzyme 4. Dieselzyme 4 has greatly improved thermal stability, with a
30-fold increase in the half-inactivation time at 50[degree sign]C relative to
the wild-type enzyme. The evolved enzyme also has dramatically increased
methanol tolerance, showing a 50-fold longer half-inactivation time in 50%
aqueous methanol. The immobilized Dieselzyme 4 enzyme retains the ability to
synthesize biodiesel and has improved longevity over wild-type or the
industrially used Brukholderia cepacia lipase during many cycles of biodiesel
synthesis. A crystal structure of Dieselzyme 4 reveals additional hydrogen bonds
and salt bridges in Dieselzyme 4 compared to the wild-type enzyme, suggesting
that polar interactions may become particularly stabilizing in the reduced
dielectric environment of the oil and methanol mixture used for biodiesel
synthesis. CONCLUSIONS: Directed evolution was used to produce a stable lipase,
Dieselzyme 4, which could be immobilized and re-used for biodiesel synthesis.
Dieselzyme 4 outperforms the industrially used lipase from Burkholderia cepacia
and provides a platform for still further evolution of desirable biodiesel
production properties.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

