 |
PDBsum entry 4hk5
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C, D:
E.C.4.1.1.66
- uracil-5-carboxylate decarboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
uracil 5-carboxylate + H+ = uracil + CO2
|
 |
 |
 |
 |
 |
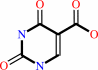 uracil 5-carboxylate
uracil 5-carboxylate
|
+
|
H(+)
|
=
|
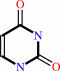 uracil
uracil
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell Res
23:1296-1309
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of isoorotate decarboxylases reveal a novel catalytic mechanism of 5-carboxyl-uracil decarboxylation and shed light on the search for DNA decarboxylase.
|
|
S.Xu,
W.Li,
J.Zhu,
R.Wang,
Z.Li,
G.L.Xu,
J.Ding.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
DNA methylation and demethylation regulate many crucial biological processes in
mammals and are linked to many diseases. Active DNA demethylation is believed to
be catalyzed by TET proteins and a putative DNA decarboxylase that may share
some similarities in sequence, structure and catalytic mechanism with isoorotate
decarboxylase (IDCase) that catalyzes decarboxylation of 5caU to U in fungi. We
report here the structures of wild-type and mutant IDCases from Cordyceps
militaris and Metarhizium anisopliae in apo form or in complexes with 5caU, U,
and an inhibitor 5-nitro-uracil. IDCases adopt a typical (β/α)8 barrel fold of
the amidohydrolase superfamily and function as dimers. A Zn(2+) is bound at the
active site and coordinated by four strictly conserved residues, one Asp and
three His. The substrate is recognized by several strictly conserved residues.
The functional roles of the key residues at the active site are validated by
mutagenesis and biochemical studies. Based on the structural and biochemical
data, we present for the first time a novel catalytic mechanism of
decarboxylation for IDCases, which might also apply to other members of the
amidohydrolase superfamily. In addition, our biochemical data show that IDCases
can catalyze decarboxylation of 5caC to C albeit with weak activity, which is
the first in vitro evidence for direct decarboxylation of 5caC to C by an
enzyme. These findings are valuable in the identification of potential DNA
decarboxylase in mammals.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

