 |
PDBsum entry 4fe3

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Protein binding
|
PDB id
|
|

|

|
4fe3
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Protein binding
|
 |
|
Title:
|
 |
Structure of murine cytosolic 5'-nucleotidase iii complexed with uridinine monophosphate
|
|
Structure:
|
 |
Cytosolic 5'-nucleotidase 3. Chain: a. Fragment: unp residues 42-327. Synonym: cytosolic 5'-nucleotidase iii, cn-iii, lupin, pyrimidine 5'- nucleotidase 1, p5'n-1, p5n-1, pn-i. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Mus musculus. Mouse. Organism_taxid: 10090. Gene: nt5c3, nt5c3_predicted, rcg_52382. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
1.74Å
|
R-factor:
|
0.172
|
R-free:
|
0.219
|
|
|
Authors:
|
 |
E.Bitto,C.A.Bingman
|
|
Key ref:
|
 |
C.L.Grobosky
et al.
(2012).
Structural basis of substrate specificity and selectivity of murine cytosolic 5'-nucleotidase III.
J Mol Biol,
423,
540-554.
PubMed id:
|
 |
|
Date:
|
 |
|
29-May-12
|
Release date:
|
29-Aug-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9D020
(5NT3A_MOUSE) -
Cytosolic 5'-nucleotidase 3A from Mus musculus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
331 a.a.
291 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 6 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.3.1.3.5
- 5'-nucleotidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-phosphate + H2O = a ribonucleoside + phosphate
|
 |
 |
 |
 |
 |
ribonucleoside 5'-phosphate
|
+
|
H2O
|
=
|
ribonucleoside
Bound ligand (Het Group name = )
matches with 40.91% similarity
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.1.3.91
- 7-methylguanosine nucleotidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
N7-methyl-GMP + H2O = N7-methylguanosine + phosphate
|
|
2.
|
CMP + H2O = cytidine + phosphate
|
|
 |
 |
 |
 |
 |
N(7)-methyl-GMP
|
+
|
H2O
|
=
|
N(7)-methylguanosine
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
CMP
Bound ligand (Het Group name = )
matches with 90.91% similarity
|
+
|
H2O
|
=
|
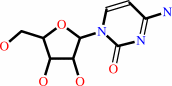 cytidine
cytidine
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Mol Biol
423:540-554
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis of substrate specificity and selectivity of murine cytosolic 5'-nucleotidase III.
|
|
C.L.Grobosky,
J.B.Lopez,
S.Rennie,
D.J.Skopelitis,
A.T.Wiest,
C.A.Bingman,
E.Bitto.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cytosolic 5'-nucleotidase III (cN-III) is responsible for selective degradation
of pyrimidine 5'-monoribonucleotides during maturation of reticulocytes to
erythrocytes. The lack of this enzymatic activity due to genetic aberrations or
lead poisoning results in a mild to moderate nonspherocytic hemolytic anemia. In
affected individuals, pyrimidine nucleotides as well as their precursor polymers
and their off-path metabolites accumulate in erythrocytes, interfering with
their proper function in ways that are not yet fully understood. This report
describes the first X-ray structure of a catalytically inactivated variant of
murine cN-III with a natural substrate, uridine 5'-monophosphate, in the active
site at 1.74Å resolution. The structure captures in an atomic detail the closed
conformation that cN-III adopts upon substrate binding. Structure and sequence
analysis coupled with enzymatic characterization of several mutants confirmed
that the aromatic ring of a nitrogenous base of substrate nucleotide is
stabilized by parallel π-stacking interactions with conserved aromatic rings of
Trp113 and His68. The nitrogenous base is further stabilized by T-shaped
stacking with the conserved aromatic ring of Tyr114, as well as by polar
contacts with side chains of Thr66 and Ser117. Two water molecules help to
stabilize the nucleotide binding by bridging it to protein residues Asp72 and
His68 via hydrogen bonds. Finally, fully conserved Glu96 is responsible for
recognition of ribose ring via two hydrogen bonds. The presented substrate
complex structure elucidates how cN-III achieves specificity for pyrimidine
5'-nucleotides and how it selects against purine 5'-nucleotides.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

