 |
PDBsum entry 4e2f

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/protein binding
|
PDB id
|
|

|

|
4e2f
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|





 (+ 0 more)
310 a.a.
(+ 0 more)
310 a.a.
|
 |

|

|

|

|

|

|

|





 (+ 0 more)
144 a.a.
(+ 0 more)
144 a.a.
|
 |

|

|
|
|
|
|
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase/protein binding
|
 |
|
Title:
|
 |
Crystal structure of e. Coli aspartate transcarbamoylase k164e/e239k mutant in an intermediate state
|
|
Structure:
|
 |
Aspartate carbamoyltransferase catalytic chain. Chain: i, k, g, c, a, e. Synonym: aspartate transcarbamylase, atcase. Engineered: yes. Mutation: yes. Aspartate carbamoyltransferase regulatory chain. Chain: d, b, j, l, h, f. Engineered: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 83333. Strain: k12. Gene: b4245, jw4204, pyrb. Expressed in: escherichia coli. Expression_system_taxid: 562. Gene: b4244, jw4203, pyri.
|
|
Resolution:
|
 |
|
2.80Å
|
R-factor:
|
0.217
|
R-free:
|
0.274
|
|
|
Authors:
|
 |
W.Guo,E.R.Kantrowitz
|
|
Key ref:
|
 |
W.Guo
et al.
(2012).
Trapping and structure determination of an intermediate in the allosteric transition of aspartate transcarbamoylase.
Proc Natl Acad Sci U S A,
109,
7741-7746.
PubMed id:
|
 |
|
Date:
|
 |
|
08-Mar-12
|
Release date:
|
02-May-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains I, K, G, C, A, E:
E.C.2.1.3.2
- aspartate carbamoyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Pyrimidine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
carbamoyl phosphate + L-aspartate = N-carbamoyl-L-aspartate + phosphate + H+
|
 |
 |
 |
 |
 |
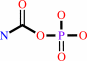 carbamoyl phosphate
carbamoyl phosphate
|
+
|
 L-aspartate
L-aspartate
|
=
|
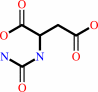 N-carbamoyl-L-aspartate
N-carbamoyl-L-aspartate
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains D, B, J, L, H, F:
E.C.?
|
|
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Proc Natl Acad Sci U S A
109:7741-7746
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Trapping and structure determination of an intermediate in the allosteric transition of aspartate transcarbamoylase.
|
|
W.Guo,
J.M.West,
A.S.Dutton,
H.Tsuruta,
E.R.Kantrowitz.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

