 |
PDBsum entry 4y2b

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/hydrolase inhibitor
|
PDB id
|
|

|

|
4y2b
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase/hydrolase inhibitor
|
 |
|
Title:
|
 |
Co-crystal structure of 3-ethyl-2-(isopropylamino)-7-(pyridin-3-yl) thieno[3,2-d]pyrimidin-4(3h)-one bound to pde7a
|
|
Structure:
|
 |
High affinity camp-specific 3',5'-cyclic phosphodiesterase 7a. Chain: a. Fragment: unp residues 130-482. Synonym: hcp1,tm22. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: pde7a. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.20Å
|
R-factor:
|
0.160
|
R-free:
|
0.196
|
|
|
Authors:
|
 |
Y.Endo,K.Kawai,T.Asano,S.Amano,Y.Asanuma,K.Sawada,Y.Onodera,N.Ueo, N.Takahashi,Y.Sonoda,N.Kamei,T.Irie
|
|
Key ref:
|
 |
Y.Endo
et al.
(2015).
2-(Isopropylamino)thieno[3,2-d]pyrimidin-4(3H)-one derivatives as selective phosphodiesterase 7 inhibitors with potent in vivo efficacy.
Bioorg Med Chem Lett,
25,
1910-1914.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
09-Feb-15
|
Release date:
|
08-Apr-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q13946
(PDE7A_HUMAN) -
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
482 a.a.
318 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.4.53
- 3',5'-cyclic-AMP phosphodiesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3',5'-cyclic AMP + H2O = AMP + H+
|
 |
 |
 |
 |
 |
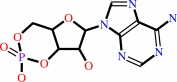 3',5'-cyclic AMP
3',5'-cyclic AMP
|
+
|
H2O
|
=
|
 AMP
AMP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Bioorg Med Chem Lett
25:1910-1914
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
2-(Isopropylamino)thieno[3,2-d]pyrimidin-4(3H)-one derivatives as selective phosphodiesterase 7 inhibitors with potent in vivo efficacy.
|
|
Y.Endo,
K.Kawai,
T.Asano,
S.Amano,
Y.Asanuma,
K.Sawada,
Y.Onodera,
N.Ueo,
N.Takahashi,
Y.Sonoda,
N.Kamei,
T.Irie.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A new series of thienopyrimidinones is synthesized and evaluated as selective
phosphodiesterase 7 (PDE7) inhibitors for the treatment of inflammatory
diseases. The modification of the substituents on thienopyrimidinone revealed
that an isopropylamino group at the 2-position was favorable for aqueous
solubility. The introduction of 3-pyrrolidines at the 7-position resulted in
good solubility, highly potent activity, and good PDE7 selectivity. Among the
synthesized compounds, compound 46 exhibited the greatest inhibition of ear
edema in a phorbol ester-induced mouse model.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

