 |
PDBsum entry 4x4r

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
RNA binding protein
|
PDB id
|
|

|

|
4x4r
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
RNA binding protein
|
 |
|
Title:
|
 |
Crystal structure of the a.Fulgidus cca-adding enzyme in complex with a g70a arginyl-tRNA minihelix ending in ccacc and ampcpp
|
|
Structure:
|
 |
Cca-adding enzyme. Chain: a, c. Fragment: a. Fulgidus cca-adding enzyme. Synonym: cca tRNA nucleotidyltransferase,tRNA cca-pyrophosphorylase, tRNA adenylyl-/cytidylyl- transferase,tRNA nucleotidyltransferase, tRNA-nt. Engineered: yes. G70a tRNA minihelix ending in ccacc. Chain: b, d.
|
|
Source:
|
 |
Archaeoglobus fulgidus. Organism_taxid: 224325. Strain: atcc 49558 / vc-16 / dsm 4304 / jcm 9628 / nbrc 100126. Gene: cca, af_2156. Expressed in: escherichia coli. Expression_system_taxid: 562. Synthetic: yes. Homo sapiens. Organism_taxid: 9606.
|
|
Resolution:
|
 |
|
3.20Å
|
R-factor:
|
0.212
|
R-free:
|
0.266
|
|
|
Authors:
|
 |
C.-D.Kuhn,L.Joshua-Tor
|
|
Key ref:
|
 |
C.D.Kuhn
et al.
(2015).
On-enzyme refolding permits small RNA and tRNA surveillance by the CCA-adding enzyme.
Cell,
160,
644-658.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
03-Dec-14
|
Release date:
|
11-Feb-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O28126
(CCA_ARCFU) -
CCA-adding enzyme from Archaeoglobus fulgidus (strain ATCC 49558 / DSM 4304 / JCM 9628 / NBRC 100126 / VC-16)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
437 a.a.
443 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|

|

|
|
|
|
G-G-C-C-G-C-G-G-C-A-G-G-U-U-C-G-A-G-U-C-C-U-G-C-C-G-C-G-C-G-C-C-A-C-C
35 bases
|
|
|
|
G-G-C-C-G-C-G-G-C-A-G-G-U-U-C-G-A-U-C-C-U-G-C-C-G-C-C-A-C-C
30 bases
|
|

|
 |
 |
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.7.72
- Cca tRNA nucleotidyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a tRNA precursor + 2 CTP + ATP = a tRNA with a 3' CCA end + 3 diphosphate
|
 |
 |
 |
 |
 |
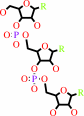 tRNA precursor
tRNA precursor
|
+
|
2
×
CTP
Bound ligand (Het Group name = )
matches with 55.00% similarity
|
+
|
 ATP
ATP
|
=
|
 tRNA with a 3' CCA end
tRNA with a 3' CCA end
|
+
|
 3
×
diphosphate
3
×
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Cell
160:644-658
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
On-enzyme refolding permits small RNA and tRNA surveillance by the CCA-adding enzyme.
|
|
C.D.Kuhn,
J.E.Wilusz,
Y.Zheng,
P.A.Beal,
L.Joshua-Tor.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Transcription in eukaryotes produces a number of long noncoding RNAs (lncRNAs).
Two of these, MALAT1 and Menβ, generate a tRNA-like small RNA in addition to
the mature lncRNA. The stability of these tRNA-like small RNAs and bona fide
tRNAs is monitored by the CCA-adding enzyme. Whereas CCA is added to stable
tRNAs and tRNA-like transcripts, a second CCA repeat is added to certain
unstable transcripts to initiate their degradation. Here, we characterize how
these two scenarios are distinguished. Following the first CCA addition cycle,
nucleotide binding to the active site triggers a clockwise screw motion,
producing torque on the RNA. This ejects stable RNAs, whereas unstable RNAs are
refolded while bound to the enzyme and subjected to a second CCA catalytic
cycle. Intriguingly, with the CCA-adding enzyme acting as a molecular vise, the
RNAs proofread themselves through differential responses to its interrogation
between stable and unstable substrates.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

