 |
PDBsum entry 4k2b
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of ntda from bacillus subtilis in complex with the internal aldimine
|
|
Structure:
|
 |
Ntd biosynthesis operon protein ntda. Chain: a, b. Engineered: yes
|
|
Source:
|
 |
Bacillus subtilis subsp. Subtilis. Organism_taxid: 224308. Strain: 168. Gene: 939788, bsu10550, np_388936.1, ntda, yhjl. Expressed in: escherichia coli. Expression_system_taxid: 511693.
|
|
Resolution:
|
 |
|
2.31Å
|
R-factor:
|
0.163
|
R-free:
|
0.214
|
|
|
Authors:
|
 |
K.E.Van Straaten,D.R.J.Palmer,D.A.R.Sanders
|
|
Key ref:
|
 |
K.E.van Straaten
et al.
(2013).
The structure of NtdA, a sugar aminotransferase involved in the kanosamine biosynthetic pathway in Bacillus subtilis, reveals a new subclass of aminotransferases.
J Biol Chem,
288,
34121-34130.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
08-Apr-13
|
Release date:
|
16-Oct-13
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O07566
(NTDA_BACSU) -
3-oxo-glucose-6-phosphate:glutamate aminotransferase from Bacillus subtilis (strain 168)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
441 a.a.
437 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.104
- 3-dehydro-glucose-6-phosphate--glutamate transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3-dehydro-D-glucose 6-phosphate + L-glutamate = D-kanosamine 6-phosphate + 2-oxoglutarate
|
 |
 |
 |
 |
 |
3-dehydro-D-glucose 6-phosphate
|
+
|
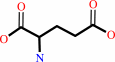 L-glutamate
L-glutamate
|
=
|
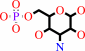 D-kanosamine 6-phosphate
D-kanosamine 6-phosphate
|
+
|
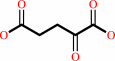 2-oxoglutarate
2-oxoglutarate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
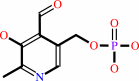 Pyridoxal 5'-phosphate
Pyridoxal 5'-phosphate
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
288:34121-34130
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of NtdA, a sugar aminotransferase involved in the kanosamine biosynthetic pathway in Bacillus subtilis, reveals a new subclass of aminotransferases.
|
|
K.E.van Straaten,
J.B.Ko,
R.Jagdhane,
S.Anjum,
D.R.Palmer,
D.A.Sanders.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
NtdA from Bacillus subtilis is a sugar aminotransferase that catalyzes the
pyridoxal phosphate-dependent equatorial transamination of 3-oxo-α-d-glucose
6-phosphate to form α-d-kanosamine 6-phosphate. The crystal structure of NtdA
shows that NtdA shares the common aspartate aminotransferase fold (Type 1) with
residues from both monomers forming the active site. The crystal structures of
NtdA alone, co-crystallized with the product α-d-kanosamine 6-phosphate, and
incubated with the amine donor glutamate reveal three key structures in the
mechanistic pathway of NtdA. The structure of NtdA alone reveals the internal
aldimine form of NtdA with the cofactor pyridoxal phosphate covalently attached
to Lys-247. The addition of glutamate results in formation of pyridoxamine
phosphate. Co-crystallization with kanosamine 6-phosphate results in the
formation of the external aldimine. Only α-d-kanosamine 6-phosphate is observed
in the active site of NtdA, not the β-anomer. A comparison of the structure and
sequence of NtdA with other sugar aminotransferases enables us to propose that
the VIβ family of aminotransferases should be divided into subfamilies based on
the catalytic lysine motif.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

