 |
PDBsum entry 4h5e

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/transferase inhibitor
|
PDB id
|
|

|

|
4h5e
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase/transferase inhibitor
|
 |
|
Title:
|
 |
Crystal structure of human fpps in ternary complex with ys0470 and isopentenyl pyrophosphate
|
|
Structure:
|
 |
Farnesyl pyrophosphate synthase. Chain: f. Fragment: unp residues 67-419. Synonym: fpp synthase, fps, (2e,6e)-farnesyl diphosphate synthase, dimethylallyltranstransferase, farnesyl diphosphate synthase, geranyltranstransferase. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: fdps, fps, kiaa1293. Expressed in: escherichia coli. Expression_system_taxid: 562
|
|
Resolution:
|
 |
|
2.04Å
|
R-factor:
|
0.188
|
R-free:
|
0.233
|
|
|
Authors:
|
 |
J.Park,Y.-S.Lin,Y.S.Tsantrizos,A.M.Berghuis
|
|
Key ref:
|
 |
J.Park
et al.
(2012).
Ternary complex structures of human farnesyl pyrophosphate synthase bound with a novel inhibitor and secondary ligands provide insights into the molecular details of the enzyme's active site closure.
Bmc Struct Biol,
12,
32.
PubMed id:
|
 |
|
Date:
|
 |
|
18-Sep-12
|
Release date:
|
26-Dec-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P14324
(FPPS_HUMAN) -
Farnesyl pyrophosphate synthase from Homo sapiens
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
419 a.a.
346 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 1:
|
 |
E.C.2.5.1.1
- dimethylallyltranstransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Terpenoid biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + dimethylallyl diphosphate = (2E)- geranyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
isopentenyl diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
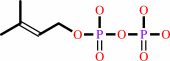 dimethylallyl diphosphate
dimethylallyl diphosphate
|
=
|
(2E)- geranyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.2.5.1.10
- (2E,6E)-farnesyl diphosphate synthase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
|
 |
 |
 |
 |
 |
Reaction:
|
 |
isopentenyl diphosphate + (2E)-geranyl diphosphate = (2E,6E)-farnesyl diphosphate + diphosphate
|
 |
 |
 |
 |
 |
 isopentenyl diphosphate
isopentenyl diphosphate
|
+
|
(2E)-geranyl diphosphate
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
 (2E,6E)-farnesyl diphosphate
(2E,6E)-farnesyl diphosphate
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Bmc Struct Biol
12:32
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Ternary complex structures of human farnesyl pyrophosphate synthase bound with a novel inhibitor and secondary ligands provide insights into the molecular details of the enzyme's active site closure.
|
|
J.Park,
Y.S.Lin,
J.W.De Schutter,
Y.S.Tsantrizos,
A.M.Berghuis.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
ABSTRACT: BACKGROUND: Human farnesyl pyrophosphate synthase (FPPS) controls
intracellular levels of farnesyl pyrophosphate, which is essential for various
biological processes. Bisphosphonate inhibitors of human FPPS are valuable
therapeutics for the treatment of bone-resorption disorders and have also
demonstrated efficacy in multiple tumor types. Inhibition of human FPPS by
bisphosphonates in vivo is thought to involve closing of the enzyme's C-terminal
tail induced by the binding of the second substrate isopentenyl pyrophosphate
(IPP). This conformational change, which occurs through a yet unclear mechanism,
seals off the enzyme's active site from the solvent environment and is essential
for catalysis. The crystal structure of human FPPS in complex with a novel
bisphosphonate YS0470 and in the absence of a second substrate showed partial
ordering of the tail in the closed conformation. RESULTS: We have determined
crystal structures of human FPPS in ternary complex with YS0470 and the
secondary ligands inorganic phosphate (Pi), inorganic pyrophosphate (PPi), and
IPP. Binding of PPi or IPP to the enzyme-inhibitor complex, but not that of Pi,
resulted in full ordering of the C-terminal tail, which is most notably
characterized by the anchoring of R351 side chain to the main frame of the
enzyme. Isothermal titration calorimetry experiments demonstrated that PPi binds
more tightly to the enzyme-inhibitor complex than IPP, and differential scanning
fluorometry experiments confirmed that Pi binding does not induce the tail
ordering. Structure analysis identified a cascade of conformational changes
required for the C-terminal tail rigidification involving Y349, F238, and Q242.
The residues K57 and N59 upon PPi/IPP binding undergo subtler conformational
changes, which may initiate this cascade. CONCLUSIONS: In human FPPS, Y349
functions as a safety switch that prevents any futile C-terminal closure and is
locked in the "off" position in the absence of bound IPP. Q242 plays
the role of a gatekeeper and directly controls the anchoring of R351 side chain.
The interactions between the residues K57 and N59 and those upstream and
downstream of Y349 are likely responsible for the switch activation. The
findings of this study can be exploited for structure-guided optimization of
existing inhibitors as well as development of new pharmacophores.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

