 |
PDBsum entry 4ghc

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
4ghc
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Structure of y257f variant of homoprotocatechuate 2,3-dioxygenase from b.Fuscum at 1.55 ang resolution
|
|
Structure:
|
 |
Homoprotocatechuate 2,3-dioxygenase. Chain: a, b, c, d. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Brevibacterium fuscum. Organism_taxid: 47914. Strain: atcc 15993. Gene: hpcd. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
1.55Å
|
R-factor:
|
0.119
|
R-free:
|
0.148
|
|
|
Authors:
|
 |
E.G.Kovaleva,J.D.Lipscomb
|
|
Key ref:
|
 |
E.G.Kovaleva
and
J.D.Lipscomb
(2012).
Structural basis for the role of tyrosine 257 of homoprotocatechuate 2,3-dioxygenase in substrate and oxygen activation.
Biochemistry,
51,
8755-8763.
PubMed id:
|
 |
|
Date:
|
 |
|
07-Aug-12
|
Release date:
|
31-Oct-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q45135
(Q45135_9MICO) -
Homoprotocatechuate 2,3-dioxygenase from Brevibacterium fuscum
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
365 a.a.
360 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.13.11.15
- 3,4-dihydroxyphenylacetate 2,3-dioxygenase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3,4-dihydroxyphenylacetate + O2 = 2-hydroxy-5-carboxymethylmuconate semialdehyde + H+
|
 |
 |
 |
 |
 |
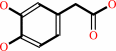 3,4-dihydroxyphenylacetate
3,4-dihydroxyphenylacetate
|
+
|
 O2
O2
|
=
|
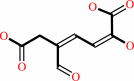 2-hydroxy-5-carboxymethylmuconate semialdehyde
2-hydroxy-5-carboxymethylmuconate semialdehyde
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe cation
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
51:8755-8763
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis for the role of tyrosine 257 of homoprotocatechuate 2,3-dioxygenase in substrate and oxygen activation.
|
|
E.G.Kovaleva,
J.D.Lipscomb.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Homoprotocatechuate 2,3-dioxygenase (FeHPCD) utilizes an active site Fe(II) to
activate O(2) in a reaction cycle that ultimately results in aromatic ring
cleavage. Here, the roles of the conserved active site residue Tyr257 are
investigated by solving the X-ray crystal structures of the Tyr257-to-Phe
variant (Y257F) in complex with the substrate homoprotocatechuate (HPCA) and the
alternative substrate 4-nitrocatechol (4NC). These are compared with structures
of the analogous wild type enzyme complexes. In addition, the oxy intermediate
of the reaction cycle of Y257F-4NC + O(2) is formed in crystallo and
structurally characterized. It is shown that both substrates adopt a previously
unrecognized, slightly nonplanar, strained conformation affecting the geometries
of all aromatic ring carbons when bound in the FeHPCD active site. This global
deviation from planarity is not observed for the Y257F variant. In the
Y257F-4NC-oxy complex, the O(2) is bound side-on to the Fe(II), while the 4NC is
chelated in two adjacent sites. The ring of the 4NC in this complex is planar,
in contrast to the equivalent FeHPCD intermediate, which exhibits substantial
local distortion of the substrate hydroxyl moiety (C2-O(-)) that is hydrogen
bonded to Tyr257. We propose that Tyr257 induces the global and local
distortions of the substrate ring in two different ways. First, van der Waals
conflict between the Tyr257-OH substituent and the substrate C2 carbon is
relieved by adopting the globally strained structure. Second, Tyr257 stabilizes
the localized out-of-plane position of the C2-O(-) by forming a stronger
hydrogen bond as the distortion increases. Both types of distortions favor
transfer of one electron out of the substrate to form a reactive semiquinone
radical. Then, the localized distortion at substrate C2 promotes formation of
the key alkylperoxo intermediate of the cycle resulting from oxygen attack on
the activated substrate at C2, which becomes sp(3) hybridized. The inability of
Y257F to promote the distorted substrate structure may explain the observed
100-fold decrease in the rates of the O(2) activation and insertion steps of the
reaction.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

