 |
PDBsum entry 4a6r
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Crystal structure of the omega transaminase from chromobacterium violaceum in the apo form, crystallised from polyacrylic acid
|
|
Structure:
|
 |
Omega transaminase. Chain: a, b. Synonym: probable aminotransferase. Engineered: yes
|
|
Source:
|
 |
Chromobacterium violaceum. Organism_taxid: 536. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
1.35Å
|
R-factor:
|
0.135
|
R-free:
|
0.163
|
|
|
Authors:
|
 |
D.T.Logan,M.Hakansson,K.Yengo,M.Svedendahl Humble,K.Engelmark Cassimjee,B.Walse,V.Abedi,H.-J.Federsel,P.Berglund
|
|
Key ref:
|
 |
M.S.Humble
et al.
(2012).
Crystal structures of the Chromobacterium violaceumω-transaminase reveal major structural rearrangements upon binding of coenzyme PLP.
Febs J,
279,
779-792.
PubMed id:
|
 |
|
Date:
|
 |
|
08-Nov-11
|
Release date:
|
25-Jan-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q7NWG4
(Q7NWG4_CHRVO) -
Probable aminotransferase from Chromobacterium violaceum (strain ATCC 12472 / DSM 30191 / JCM 1249 / CCUG 213 / NBRC 12614 / NCIMB 9131 / NCTC 9757 / MK)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
459 a.a.
423 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.6.1.62
- adenosylmethionine--8-amino-7-oxononanoate transaminase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(8S)-8-amino-7-oxononanoate + S-adenosyl-L-methionine = S-adenosyl-4- methylsulfanyl-2-oxobutanoate + (7R,8S)-7,8-diammoniononanoate
|
 |
 |
 |
 |
 |
(8S)-8-amino-7-oxononanoate
|
+
|
 S-adenosyl-L-methionine
S-adenosyl-L-methionine
|
=
|
S-adenosyl-4- methylsulfanyl-2-oxobutanoate
|
+
|
(7R,8S)-7,8-diammoniononanoate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Pyridoxal 5'-phosphate
|
 |
 |
 |
 |
 |
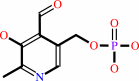 Pyridoxal 5'-phosphate
Pyridoxal 5'-phosphate
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Febs J
279:779-792
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal structures of the Chromobacterium violaceumω-transaminase reveal major structural rearrangements upon binding of coenzyme PLP.
|
|
M.S.Humble,
K.E.Cassimjee,
M.Håkansson,
Y.R.Kimbung,
B.Walse,
V.Abedi,
H.J.Federsel,
P.Berglund,
D.T.Logan.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The bacterial ω-transaminase from Chromobacterium violaceum (Cv-ωTA,
EC2.6.1.18) catalyses industrially important transamination reactions by use of
the coenzyme pyridoxal 5'-phosphate (PLP). Here, we present four crystal
structures of Cv-ωTA: two in the apo form, one in the holo form and one in an
intermediate state, at resolutions between 1.35 and 2.4 Å. The enzyme is a
homodimer with a molecular mass of ∼ 100 kDa. Each monomer has an active site
at the dimeric interface that involves amino acid residues from both subunits.
The apo-Cv-ωTA structure reveals unique 'relaxed' conformations of three
critical loops involved in structuring the active site that have not previously
been seen in a transaminase. Analysis of the four crystal structures reveals
major structural rearrangements involving elements of the large and small
domains of both monomers that reorganize the active site in the presence of PLP.
The conformational change appears to be triggered by binding of the phosphate
group of PLP. Furthermore, one of the apo structures shows a disordered
'roof ' over the PLP-binding site, whereas in the other apo form and the holo
form the 'roof' is ordered. Comparison with other known transaminase crystal
structures suggests that ordering of the 'roof' structure may be associated with
substrate binding in Cv-ωTA and some other transaminases. DATABASE: The atomic
coordinates and structure factors for the Chromobacterium
violaceumω-transaminase crystal structures can be found in the RCSB Protein
Data Bank (http://www.rcsb.org) under the accession codes 4A6U for the
holoenzyme, 4A6R for the apo1 form, 4A6T for the apo2 form and 4A72 for the
mixed form STRUCTURED DIGITAL ABSTRACT: • -transaminases and -transaminases
bind by dynamic light scattering (View interaction) • -transaminase and
-transaminase bind by x-ray crystallography (View interaction) • -transaminase
and -transaminase bind by x-ray crystallography (View interaction).

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

