 |
PDBsum entry 4a1t

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Hydrolase/peptide
|
PDB id
|
|

|

|
4a1t
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase/peptide
|
 |
|
Title:
|
 |
Co-complex of the of ns3-4a protease with the inhibitory peptide cp5- 46-a (in-house data)
|
|
Structure:
|
 |
Nonstructural protein 4a, serine protease ns3. Chain: a, b. Fragment: residues 1678-1690,1028-1206. Synonym: ns3-4a protease, ns4a, hepacivirin, ns3p, p70. Engineered: yes. Other_details: fusion protein of 4a (1678-1690) with ns3 (1028-1206). Cp5-46-a peptide. Chain: c, d. Engineered: yes.
|
|
Source:
|
 |
Hepatitis c virus. Organism_taxid: 11103. Strain: 1b. Expressed in: escherichia coli. Expression_system_taxid: 469008. Expression_system_variant: rosetta2. Other_details: hcv replicon i389/ns3-3'utr (aj242654). Synthetic: yes. Synthetic construct.
|
|
Resolution:
|
 |
|
2.05Å
|
R-factor:
|
0.198
|
R-free:
|
0.245
|
|
|
Authors:
|
 |
S.Schmelz,J.Kuegler,J.Collins,D.W.Heinz
|
|
Key ref:
|
 |
J.Kügler
et al.
(2012).
High affinity peptide inhibitors of the hepatitis C virus NS3-4A protease refractory to common resistant mutants.
J Biol Chem,
287,
39224-39232.
PubMed id:
|
 |
|
Date:
|
 |
|
19-Sep-11
|
Release date:
|
19-Sep-12
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P26662
(POLG_HCVJA) -
Genome polyprotein from Hepatitis C virus genotype 1b (isolate Japanese)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
3010 a.a.
193 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B:
E.C.2.7.7.48
- RNA-directed Rna polymerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
RNA(n) + a ribonucleoside 5'-triphosphate = RNA(n+1) + diphosphate
|
 |
 |
 |
 |
 |
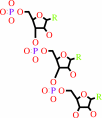 RNA(n)
RNA(n)
|
+
|
ribonucleoside 5'-triphosphate
|
=
|
RNA(n+1)
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains A, B:
E.C.3.4.21.98
- hepacivirin.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Hydrolysis of four peptide bonds in the viral precursor polyprotein, commonly with Asp or Glu in the P6 position, Cys or Thr in P1 and Ser or Ala in P1'.
|
 |
 |
 |
 |
 |
Enzyme class 4:
|
 |
Chains A, B:
E.C.3.4.22.-
- ?????
|
|
 |
 |
 |
 |
 |
Enzyme class 5:
|
 |
Chains A, B:
E.C.3.6.1.15
- nucleoside-triphosphate phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a ribonucleoside 5'-triphosphate + H2O = a ribonucleoside 5'-diphosphate + phosphate + H+
|
 |
 |
 |
 |
 |
ribonucleoside 5'-triphosphate
|
+
|
H2O
|
=
|
ribonucleoside 5'-diphosphate
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 6:
|
 |
Chains A, B:
E.C.3.6.4.13
- Rna helicase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
ATP + H2O = ADP + phosphate + H+
|
 |
 |
 |
 |
 |
 ATP
ATP
|
+
|
H2O
|
=
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Chem
287:39224-39232
(2012)
|
|
PubMed id:
|
|
|
|

|
| |
|
High affinity peptide inhibitors of the hepatitis C virus NS3-4A protease refractory to common resistant mutants.
|
|
J.Kügler,
S.Schmelz,
J.Gentzsch,
S.Haid,
E.Pollmann,
J.van den Heuvel,
R.Franke,
T.Pietschmann,
D.W.Heinz,
J.Collins.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Hepatitis C virus (HCV) NS3-4A protease is essential for viral replication. All
current small molecular weight drugs against NS3-4A are substrate
peptidomimetics that have a similar binding and resistance profile. We developed
inhibitory peptides (IPs) capping the active site and binding via a novel
"tyrosine" finger at an alternative NS3-4A site that is of particular
interest for further HCV drug development. The peptides are not cleaved due to a
combination of geometrical constraints and impairment of the oxyanion hole
function. Selection and optimization through combinatorial phagemid display,
protein crystallography, and further modifications resulted in a 32-amino acid
peptide with a K(i) of 0.53 nm. Inhibition of viral replication in cell culture
was demonstrated by fusion to a cell-penetrating peptide. Negligible
susceptibility to known (A156V and R155K) resistance mutations of the NS3-4A
protease was observed. This work shows for the first time that antiviral
peptides can target an intracellular site and reveals a novel druggable site on
the HCV protease.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| |

