 |
PDBsum entry 3ftf

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Transferase/RNA
|
PDB id
|
|

|

|
3ftf
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.1.1.182
- 16S rRNA (adenine(1518)-N(6)/adenine(1519)-N(6))-dimethyltransferase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
adenosine1518/adenosine1519 in 16S rRNA + 4 S-adenosyl-L-methionine = N6-dimethyladenosine1518/N6-dimethyladenosine1519 in 16S rRNA + 4 S-adenosyl-L-homocysteine + 4 H+
|
 |
 |
 |
 |
 |
adenosine(1518)/adenosine(1519) in 16S rRNA
|
+
|
 4
×
S-adenosyl-L-methionine
4
×
S-adenosyl-L-methionine
|
=
|
N(6)-dimethyladenosine(1518)/N(6)-dimethyladenosine(1519) in 16S rRNA
|
+
|
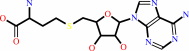 4
×
S-adenosyl-L-homocysteine
4
×
S-adenosyl-L-homocysteine
|
+
|
4
×
H(+)
Bound ligand (Het Group name = )
corresponds exactly
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
17:374-385
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural Basis for Binding of RNA and Cofactor by a KsgA Methyltransferase.
|
|
C.Tu,
J.E.Tropea,
B.P.Austin,
D.L.Court,
D.S.Waugh,
X.Ji.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Among methyltransferases, KsgA and the reaction it catalyzes are conserved
throughout evolution. However, the specifics of substrate recognition by the
enzyme remain unknown. Here we report structures of Aquifex aeolicus KsgA, in
its ligand-free form, in complex with RNA, and in complex with both RNA and
S-adenosylhomocysteine (SAH, reaction product of cofactor S-adenosylmethionine),
revealing critical structural information on KsgA-RNA and KsgA-SAH interactions.
Moreover, the structures show how conformational changes that occur upon RNA
binding create the cofactor-binding site. There are nine conserved functional
motifs (motifs I-VIII and X) in KsgA. Prior to RNA binding, motifs I and VIII
are flexible, each exhibiting two distinct conformations. Upon RNA binding, the
two motifs become stabilized in one of these conformations, which is compatible
with the binding of SAH. Motif X, which is also stabilized upon RNA binding, is
directly involved in the binding of SAH.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Ligand-free Aa-KsgA Structures
(A) Stereo view
showing the ribbon diagram of the KsgA1 structure. The α
helices, β strands, and 3[10] helices are numbered and shown in
cyan, magenta, and blue, respectively.
(B) Cα
superposition of KsgA1 (yellow), KsgA2 (magenta), Ec-KsgA-chain
A (cyan; PDB ID code 1QYR), and Ec-KsgA-chain B (green, PDB
entry 1QYR).
(C) Two distinct conformations of motif I.
(D) Two distinct conformations of motif VIII.
|
 |
Figure 6.
Figure 6. The Putative Catalytic Center of KsgA
(A)
Stereo view showing the superposition of tetraloop structures
with the KsgA-RNA-SAH complex. SAH is shown as spheres (carbon
in gray, nitrogen in blue, oxygen in red, and sulfur in yellow).
KsgA is shown as a ribbon diagram in gray. RNAs are illustrated
as tube-and-stick models with the positions of A1518 and A1519
indicated by spheres. RNA in KsgA-RNA-SAH is shown in orange, a
structure of product RNA with dimethylated A1518 and A1519 (PDB
ID code 2AW7) in cyan, and a model for substrate RNA with
unmethylated adenosines (PDB ID code 1AFX) in magenta.
(B)
Stereo view showing the superposition of the putative catalytic
center of KsgA as observed in the ternary KsgA-RNA-SAH complex
(green) with the catalytic center assembly of M·TaqI as
observed in the M·TaqI-DNA-NEA structure (PDB ID code
1G38; yellow). The alignment was based on the cofactor SAH
(stick model with carbon in green, nitrogen in blue, oxygen in
red, and sulfur in yellow) and the cofactor analog NEA (stick
model with carbon in yellow). The target adenine dA606 for
M·TaqI is shown as a stick model in magenta, whereas the
A1518 and A1519 for KsgA are shown as stick models in red. The
contact between atom N6 of dA606 in the M·TaqI complex
and atom S of SAH in the KsgA complex is indicated with a dashed
line in black.
(C) A close-up stereo view showing the
details of the adenine-binding site. The amino acid side chains
in motif IV (N105, P106, P107, and Y108) and motif VIII (F196)
in M·TaqI are shown as line models in yellow. The
corresponding side chains in KsgA (N101, L102, P103, Y104, and
F166) are shown in green. The adenine substrate is shown in
magenta.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Structure
(2009,
17,
374-385)
copyright 2009.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Guelorget,
and
B.Golinelli-Pimpaneau
(2011).
Mechanism-based strategies for trapping and crystallizing complexes of RNA-modifying enzymes.
|
| |
Structure,
19,
282-291.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

