 |
PDBsum entry 3flc

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.1.30
- glycerol kinase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
glycerol + ATP = sn-glycerol 3-phosphate + ADP + H+
|
 |
 |
 |
 |
 |
 glycerol
glycerol
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
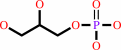 sn-glycerol 3-phosphate
sn-glycerol 3-phosphate
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
48:346-356
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural characterizations of glycerol kinase: unraveling phosphorylation-induced long-range activation.
|
|
J.I.Yeh,
R.Kettering,
R.Saxl,
A.Bourand,
E.Darbon,
N.Joly,
P.Briozzo,
J.Deutscher.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Glycerol metabolism provides a central link between sugar and fatty acid
catabolism. In most bacteria, glycerol kinase plays a crucial role in regulating
channel/facilitator-dependent uptake of glycerol into the cell. In the firmicute
Enterococcus casseliflavus, this enzyme's activity is enhanced by
phosphorylation of the histidine residue (His232) located in its activation
loop, approximately 25 A from its catalytic cleft. We reported earlier that some
mutations of His232 altered enzyme activities; we present here the crystal
structures of these mutant GlpK enzymes. The structure of a mutant enzyme with
enhanced enzymatic activity, His232Arg, reveals that residues at the catalytic
cleft are more optimally aligned to bind ATP and mediate phosphoryl transfer.
Specifically, the position of Arg18 in His232Arg shifts by approximately 1 A
when compared to its position in wild-type (WT), His232Ala, and His232Glu
enzymes. This new conformation of Arg18 is more optimally positioned at the
presumed gamma-phosphate location of ATP, close to the glycerol substrate. In
addition to structural changes exhibited at the active site, the conformational
stability of the activation loop is decreased, as reflected by an approximately
35% increase in B factors ("thermal factors") in a mutant enzyme displaying
diminished activity, His232Glu. Correlating conformational changes to alteration
of enzymatic activities in the mutant enzymes identifies distinct localized
regions that can have profound effects on intramolecular signal transduction.
Alterations in pairwise interactions across the dimer interface can communicate
phosphorylation states over 25 A from the activation loop to the catalytic
cleft, positioning Arg18 to form favorable interactions at the
beta,gamma-bridging position with ATP. This would offset loss of the hydrogen
bonds at the gamma-phosphate of ATP during phosphoryl transfer to glycerol,
suggesting that appropriate alignment of the second substrate of glycerol
kinase, the ATP molecule, may largely determine the rate of glycerol 3-phosphate
production.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.W.Pettigrew
(2009).
Oligomeric interactions provide alternatives to direct steric modes of control of sugar kinase/actin/hsp70 superfamily functions by heterotropic allosteric effectors: inhibition of E. coli glycerol kinase.
|
| |
Arch Biochem Biophys,
492,
29-39.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

