 |
PDBsum entry 3f6b

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.1.1.7
- benzoylformate decarboxylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
phenylglyoxylate + H+ = benzaldehyde + CO2
|
 |
 |
 |
 |
 |
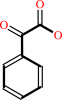 phenylglyoxylate
phenylglyoxylate
|
+
|
H(+)
|
=
|
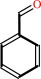 benzaldehyde
benzaldehyde
|
+
|
 CO2
CO2
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Thiamine diphosphate
|
 |
 |
 |
 |
 |
Thiamine diphosphate
Bound ligand (Het Group name =
8PA)
matches with 72.22% similarity
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
48:981-994
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Detection and time course of formation of major thiamin diphosphate-bound covalent intermediates derived from a chromophoric substrate analogue on benzoylformate decarboxylase.
|
|
S.Chakraborty,
N.S.Nemeria,
A.Balakrishnan,
G.S.Brandt,
M.M.Kneen,
A.Yep,
M.J.McLeish,
G.L.Kenyon,
G.A.Petsko,
D.Ringe,
F.Jordan.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The mechanism of the enzyme benzoylformate decarboxylase (BFDC), which carries
out a typical thiamin diphosphate (ThDP)-dependent nonoxidative decarboxylation
reaction, was studied with the chromophoric alternate substrate
(E)-2-oxo-4(pyridin-3-yl)-3-butenoic acid (3-PKB). Addition of 3-PKB resulted in
the appearance of two transient intermediates formed consecutively, the first
one to be formed a predecarboxylation ThDP-bound intermediate with lambda(max)
at 477 nm, and the second one corresponding to the first postdecarboxylation
intermediate the enamine with lambda(max) at 437 nm. The time course of
formation/depletion of the PKB-ThDP covalent complex and of the enamine showed
that decarboxylation was slower than formation of the PKB-ThDP covalent adduct.
When the product of decarboxylation 3-(pyridin-3-yl)acrylaldehyde (PAA) was
added to BFDC, again an absorbance with lambda(max) at 473 nm was formed,
corresponding to the tetrahedral adduct of PAA with ThDP. Addition of
well-formed crystals of BFDC to a solution of PAA resulted in a high resolution
(1.34 A) structure of the BFDC-bound adduct of ThDP with PAA confirming the
tetrahedral nature at the C2alpha atom, rather than of the enamine, and
supporting the assignment of the lambda(max) at 473 nm to the PAA-ThDP adduct.
The structure of the PAA-ThDP covalent complex is the first example of a
product-ThDP adduct on BFDC. Similar studies with 3-PKB indicated that
decarboxylation had taken place. Evidence was also obtained for the slow
formation of the enamine intermediate when BFDC was incubated with benzaldehyde,
the product of the decarboxylation reaction thus confirming its presence on the
reaction pathway.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
N.S.Nemeria,
S.Chakraborty,
A.Balakrishnan,
and
F.Jordan
(2009).
Reaction mechanisms of thiamin diphosphate enzymes: defining states of ionization and tautomerization of the cofactor at individual steps.
|
| |
FEBS J,
276,
2432-3446.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

