 |
PDBsum entry 3b6r

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.7.3.2
- creatine kinase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Creatine Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
creatine + ATP = N-phosphocreatine + ADP + H+
|
 |
 |
 |
 |
 |
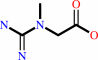 creatine
creatine
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
N-phosphocreatine
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 ADP
ADP
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Febs Lett
582:3959-3965
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural studies of human brain-type creatine kinase complexed with the ADP-Mg2+-NO3- -creatine transition-state analogue complex.
|
|
S.M.Bong,
J.H.Moon,
K.H.Nam,
K.S.Lee,
Y.M.Chi,
K.Y.Hwang.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Creatine kinase is a member of the phosphagen kinase family, which catalyzes the
reversible phosphoryl transfer reaction that occurs between ATP and creatine to
produce ADP and phosphocreatine. Here, three structural aspects of
human-brain-type-creatine-kinase (hBB-CK) were identified by X-ray
crystallography: the ligand-free-form at 2.2A; the ADP-Mg2+, nitrate, and
creatine complex (transition-state-analogue complex; TSAC); and the
ADP-Mg2+-complex at 2.0A. The structures of ligand-bound hBB-CK revealed two
different monomeric states in a single homodimer. One monomer is a closed form,
either bound to TSAC or the ADP-Mg2+-complex, and the second monomer is an
unliganded open form. These structural studies provide a detailed mechanism
indicating that the binding of ADP-Mg2+ alone may trigger conformational changes
in hBB-CK that were not observed with muscle-type-CK.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
N.Liu,
J.S.Wang,
W.D.Wang,
and
J.C.Pan
(2011).
The role of Cys271 in conformational changes of arginine kinase.
|
| |
Int J Biol Macromol,
49,
98.
|
 |
|
|
|
|
 |
Q.Xu,
and
R.L.Dunbrack
(2011).
The protein common interface database (ProtCID)--a comprehensive database of interactions of homologous proteins in multiple crystal forms.
|
| |
Nucleic Acids Res,
39,
D761-D770.
|
 |
|
|
|
|
 |
Y.S.Gao,
J.T.Su,
and
Y.B.Yan
(2010).
Sequential events in the irreversible thermal denaturation of human brain-type creatine kinase by spectroscopic methods.
|
| |
Int J Mol Sci,
11,
2584-2596.
|
 |
|
|
|
|
 |
Q.Sheng,
H.C.Zou,
Z.R.Lü,
F.Zou,
Y.D.Park,
Y.B.Yan,
and
S.J.Yao
(2009).
Effects of acrylamide on the activity and structure of human brain creatine kinase.
|
| |
Int J Mol Sci,
10,
4210-4222.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

