 |
PDBsum entry 3zlp

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
3zlp
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Crystal structure of schistosoma mansoni peroxiredoxin 1 c48p mutant form with four decamers in the asymmetric unit
|
|
Structure:
|
 |
Thioredoxin peroxidase. Chain: a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, p, q, r, s, t, u, v, w, x, y, z, a, b, c, d, e, f, g, h, i, j, k, l, m, n. Synonym: peroxiredoxin i, thioredoxin peroxidase 1. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Schistosoma mansoni. Organism_taxid: 6183. Expressed in: escherichia coli. Expression_system_taxid: 469008. Expression_system_variant: plyss.
|
|
Resolution:
|
 |
|
3.52Å
|
R-factor:
|
0.276
|
R-free:
|
0.289
|
|
|
Authors:
|
 |
F.Saccoccia,F.Angelucci,M.Ardini,G.Boumis,M.Brunori,L.Dileandro, R.Ippoliti,A.E.Miele,G.Natoli,S.Scotti,A.Bellelli
|
|
Key ref:
|
 |
F.Angelucci
et al.
(2013).
Switching between the alternative structures and functions of a 2-Cys peroxiredoxin, by site-directed mutagenesis.
J Mol Biol,
425,
4556-4568.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
04-Feb-13
|
Release date:
|
11-Sep-13
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O97161
(O97161_SCHMA) -
Thioredoxin peroxidase from Schistosoma mansoni
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
185 a.a.
163 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.11.1.15
- Transferred entry: 1.11.1.24, 1.11.1.25, 1.11.1.26, 1.11.1.27, 1.11.1.28
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Peroxiredoxin
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 R'-SH + ROOH = R'-S-S-R' + H2O + ROH
|
 |
 |
 |
 |
 |
 2
×
R'-SH
2
×
R'-SH
|
+
|
 ROOH
ROOH
|
=
|
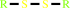 R'-S-S-R'
R'-S-S-R'
|
+
|
H(2)O
|
+
|
 ROH
ROH
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
425:4556-4568
(2013)
|
|
PubMed id:
|
|
|
|

|
| |
|
Switching between the alternative structures and functions of a 2-Cys peroxiredoxin, by site-directed mutagenesis.
|
|
F.Angelucci,
F.Saccoccia,
M.Ardini,
G.Boumis,
M.Brunori,
L.Di Leandro,
R.Ippoliti,
A.E.Miele,
G.Natoli,
S.Scotti,
A.Bellelli.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Members of the typical 2-Cys peroxiredoxin (Prx) subfamily represent an
intriguing example of protein moonlighting behavior since this enzyme shifts
function: indeed, upon chemical stimuli, such as oxidative stress, Prx undergoes
a switch from peroxidase to molecular chaperone, associated to a change in
quaternary structure from dimers/decamers to higher-molecular-weight (HMW)
species. In order to detail the structural mechanism of this switch at molecular
level, we have designed and expressed mutants of peroxiredoxin I from
Schistosoma mansoni (SmPrxI) with constitutive HMW assembly and molecular
chaperone activity. By a combination of X-ray crystallography, transmission
electron microscopy and functional experiments, we defined the structural events
responsible for the moonlighting behavior of 2-Cys Prx and we demonstrated that
acidification is coupled to local structural variations localized at the active
site and a change in oligomerization to HMW forms, similar to those induced by
oxidative stress. Moreover, we suggest that the binding site of the unfolded
polypeptide is at least in part contributed by the hydrophobic surface exposed
by the unfolding of the active site. We also find an inverse correlation between
the extent of ring stacking and molecular chaperone activity that is explained
assuming that the binding occurs at the extremities of the nanotube, and the
longer the nanotube is, the lesser the ratio binding sites/molecular mass is.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

