 |
PDBsum entry 3ll4

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Structure of the h13a mutant of ykr043c in complex with fructose-1,6- bisphosphate
|
|
Structure:
|
 |
Uncharacterized protein ykr043c. Chain: a, b. Engineered: yes. Mutation: yes
|
|
Source:
|
 |
Saccharomyces cerevisiae. Yeast. Organism_taxid: 4932. Strain: s288c. Gene: ykr043c. Expressed in: escherichia coli. Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.49Å
|
R-factor:
|
0.225
|
R-free:
|
0.266
|
|
|
Authors:
|
 |
A.Singer,X.Xu,H.Cui,A.Dong,P.J.Stogios,A.M.Edwards,A.Joachimiak, A.Savchenko,A.F.Yakunin,Midwest Center For Structural Genomics (Mcsg)
|
|
Key ref:
|
 |
E.Kuznetsova
et al.
(2010).
Structure and activity of the metal-independent fructose-1,6-bisphosphatase YK23 from Saccharomyces cerevisiae.
J Biol Chem,
285,
21049-21059.
PubMed id:
|
 |
|
Date:
|
 |
|
28-Jan-10
|
Release date:
|
09-Mar-10
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P36136
(SHB17_YEAST) -
Sedoheptulose 1,7-bisphosphatase from Saccharomyces cerevisiae (strain ATCC 204508 / S288c)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
271 a.a.
261 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.3.37
- sedoheptulose-bisphosphatase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Calvin Cycle
|
 |
 |
 |
 |
 |
Reaction:
|
 |
D-sedoheptulose 1,7-bisphosphate + H2O = D-sedoheptulose 7-phosphate + phosphate
|
 |
 |
 |
 |
 |
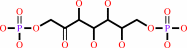 D-sedoheptulose 1,7-bisphosphate
D-sedoheptulose 1,7-bisphosphate
|
+
|
H2O
|
=
|
D-sedoheptulose 7-phosphate
Bound ligand (Het Group name = )
matches with 72.73% similarity
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Biol Chem
285:21049-21059
(2010)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure and activity of the metal-independent fructose-1,6-bisphosphatase YK23 from Saccharomyces cerevisiae.
|
|
E.Kuznetsova,
L.Xu,
A.Singer,
G.Brown,
A.Dong,
R.Flick,
H.Cui,
M.Cuff,
A.Joachimiak,
A.Savchenko,
A.F.Yakunin.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Fructose-1,6-bisphosphatase (FBPase), a key enzyme of gluconeogenesis and
photosynthetic CO(2) fixation, catalyzes the hydrolysis of fructose
1,6-bisphosphate (FBP) to produce fructose 6-phosphate, an important precursor
in various biosynthetic pathways. All known FBPases are metal-dependent enzymes,
which are classified into five different classes based on their amino acid
sequences. Eukaryotes are known to contain only the type-I FBPases, whereas all
five types exist in various combinations in prokaryotes. Here we demonstrate
that the uncharacterized protein YK23 from Saccharomyces cerevisiae efficiently
hydrolyzes FBP in a metal-independent reaction. YK23 is a member of the
histidine phosphatase (phosphoglyceromutase) superfamily with homologues found
in all organisms. The crystal structure of the YK23 apo-form was solved at
1.75-A resolution and revealed the core domain with the alpha/beta/alpha-fold
covered by two small cap domains. Two liganded structures of this protein show
the presence of two phosphate molecules (an inhibitor) or FBP (a substrate)
bound to the active site. FBP is bound in its linear, open conformation with the
cleavable C1-phosphate positioned deep in the active site. Alanine replacement
mutagenesis of YK23 identified six conserved residues absolutely required for
activity and suggested that His(13) and Glu(99) are the primary catalytic
residues. Thus, YK23 represents the first family of metal-independent FBPases
and a second FBPase family in eukaryotes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

