 |
PDBsum entry 3jd0

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
3jd0
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Glutamate dehydrogenase in complex with gtp
|
|
Structure:
|
 |
Glutamate dehydrogenase 1, mitochondrial. Chain: a, b, c, d, e, f. Fragment: unp residues 58-558. Synonym: gdh 1. Ec: 1.4.1.3
|
|
Source:
|
 |
Bos taurus. Bovine. Organism_taxid: 9913
|
|
Authors:
|
 |
M.J.Borgnia,S.Banerjee,A.Merk,D.Matthies,A.Bartesaghi,P.Rao, J.Pierson,L.A.Earl,V.Falconieri,S.Subramaniam,J.L.S.Milne
|
|
Key ref:
|
 |
M.J.Borgnia
et al.
(2016).
Using Cryo-EM to Map Small Ligands on Dynamic Metabolic Enzymes: Studies with Glutamate Dehydrogenase.
Mol Pharmacol,
89,
645-651.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
28-Mar-16
|
Release date:
|
27-Apr-16
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P00366
(DHE3_BOVIN) -
Glutamate dehydrogenase 1, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
558 a.a.
496 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.4.1.3
- glutamate dehydrogenase [NAD(P)(+)].
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
L-glutamate + NAD+ + H2O = 2-oxoglutarate + NH4+ + NADH + H+
|
|
2.
|
L-glutamate + NADP+ + H2O = 2-oxoglutarate + NH4+ + NADPH + H+
|
|
 |
 |
 |
 |
 |
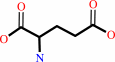 L-glutamate
L-glutamate
|
+
|
NAD(+)
Bound ligand (Het Group name = )
matches with 55.10% similarity
|
+
|
H2O
|
=
|
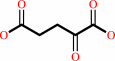 2-oxoglutarate
2-oxoglutarate
|
+
|
NH4(+)
|
+
|
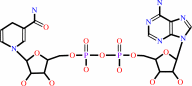 NADH
NADH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 L-glutamate
L-glutamate
|
+
|
NADP(+)
Bound ligand (Het Group name = )
matches with 50.94% similarity
|
+
|
H2O
|
=
|
 2-oxoglutarate
2-oxoglutarate
|
+
|
NH4(+)
|
+
|
 NADPH
NADPH
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Mol Pharmacol
89:645-651
(2016)
|
|
PubMed id:
|
|
|
|

|
| |
|
Using Cryo-EM to Map Small Ligands on Dynamic Metabolic Enzymes: Studies with Glutamate Dehydrogenase.
|
|
M.J.Borgnia,
S.Banerjee,
A.Merk,
D.Matthies,
A.Bartesaghi,
P.Rao,
J.Pierson,
L.A.Earl,
V.Falconieri,
S.Subramaniam,
J.L.Milne.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Cryo-electron microscopy (cryo-EM) methods are now being used to determine
structures at near-atomic resolution and have great promise in molecular
pharmacology, especially in the context of mapping the binding of small-molecule
ligands to protein complexes that display conformational flexibility. We
illustrate this here using glutamate dehydrogenase (GDH), a 336-kDa metabolic
enzyme that catalyzes the oxidative deamination of glutamate. Dysregulation of
GDH leads to a variety of metabolic and neurologic disorders. Here, we report
near-atomic resolution cryo-EM structures, at resolutions ranging from 3.2 Å to
3.6 Å for GDH complexes, including complexes for which crystal structures are
not available. We show that the binding of the coenzyme NADH alone or in concert
with GTP results in a binary mixture in which the enzyme is in either an
"open" or "closed" state. Whereas the structure of NADH in
the active site is similar between the open and closed states, it is
unexpectedly different at the regulatory site. Our studies thus demonstrate that
even in instances when there is considerable structural information available
from X-ray crystallography, cryo-EM methods can provide useful complementary
insights into regulatory mechanisms for dynamic protein complexes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

