 |
PDBsum entry 3it3

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.3.2
- acid phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a phosphate monoester + H2O = an alcohol + phosphate
|
 |
 |
 |
 |
 |
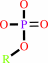 phosphate monoester
phosphate monoester
|
+
|
H2O
|
=
|
 alcohol
alcohol
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
394:893-904
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
Crystal Structures of the histidine acid phosphatase from Francisella tularensis provide insight into substrate recognition.
|
|
H.Singh,
R.L.Felts,
J.P.Schuermann,
T.J.Reilly,
J.J.Tanner.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Histidine acid phosphatases catalyze the transfer of a phosphoryl group from
phosphomonoesters to water at acidic pH using an active-site histidine. The
histidine acid phosphatase from the category A pathogen Francisella tularensis
(FtHAP) has been implicated in intramacrophage survival and virulence,
motivating interest in understanding the structure and mechanism of this enzyme.
Here, we report a structure-based study of ligand recognition by FtHAP. The
1.70-A-resolution structure of FtHAP complexed with the competitive inhibitor
l(+)-tartrate was solved using single-wavelength anomalous diffraction phasing.
Structures of the ligand-free enzyme and the complex with inorganic phosphate
were determined at resolutions of 1.85 and 1.70 A, respectively. The structure
of the Asp261Ala mutant enzyme complexed with the substrate 3'-AMP was
determined at 1.50 A resolution to gain insight into substrate recognition.
FtHAP exhibits a two-domain fold similar to that of human prostatic acid
phosphatase, consisting of an alpha/beta core domain and a smaller domain that
caps the core domain. The structures show that the core domain supplies the
phosphoryl binding site, catalytic histidine (His17), and an aspartic acid
residue (Asp261) that protonates the leaving group, while the cap domain
contributes residues that enforce substrate preference. FtHAP and human
prostatic acid phosphatase differ in the orientation of the crucial first helix
of the cap domain, implying differences in the substrate preferences of the two
enzymes. 3'-AMP binds in one end of a 15-A-long tunnel, with the adenine clamped
between Phe23 and Tyr135, and the ribose 2'-hydroxyl interacting with Gln132.
The importance of the clamp is confirmed with site-directed mutagenesis;
mutation of Phe23 and Tyr135 individually to Ala increases K(m) by factors of 7
and 10, respectively. The structural data are consistent with a role for FtHAP
in scavenging phosphate from small molecules present in host macrophage cells.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Overall fold of FtHAP. (a) Ribbon drawing of the
FtHAP protomer. Residues are colored according to a rainbow
scheme, with blue at the N-terminus and red at the C-terminus.
The P[i] ligand is shown in spheres. The side chains of His17,
Phe23, and Tyr135 are drawn as sticks in white. Strands of the
β-sheet are numbered. The first three helices of the
polypeptide chain are labeled α1, α2, and α3. The conserved
portion of the dimerization loop (residues 116–121) is colored
magenta. (b) Ribbon and surface representations of the protomer,
highlighting domain structure. The orientation is the same as in
(a). The core domain is colored blue, and the cap domain is
colored pink. The P[i] ligand is shown in spheres, and the side
chains of His17, Phe23, and Tyr135 are drawn as sticks.
|
 |
Figure 5.
Fig. 5. Cutaway view of the substrate-binding tunnel with
bound 3′-AMP.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2009,
394,
893-904)
copyright 2009.
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

