 |
PDBsum entry 3e2d

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.3.1
- alkaline phosphatase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a phosphate monoester + H2O = an alcohol + phosphate
|
 |
 |
 |
 |
 |
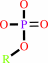 phosphate monoester
phosphate monoester
|
+
|
H2O
|
=
|
 alcohol
alcohol
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+); Zn(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochim Biophys Acta
1794:297-308
(2009)
|
|
PubMed id:
|
|
|
|

|
| |
|
The 1.4 A crystal structure of the large and cold-active Vibrio sp. alkaline phosphatase.
|
|
R.Helland,
R.L.Larsen,
B.Asgeirsson.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Alkaline phosphatase (AP) from the cold-adapted Vibrio strain G15-21 is among
the AP variants with the highest known k(cat) value. Here the structure of the
enzyme at 1.4 A resolution is reported and compared to APs from E. coli, human
placenta, shrimp and the Antarctic bacterium strain TAB5. The Vibrio AP is a
dimer although its monomers are without the long N-terminal helix that embraces
the other subunit in many other APs. The long insertion loop, previously noted
as a special feature of the Vibrio AP, serves a similar function. The surface
does not have the high negative charge density as observed in shrimp AP, but a
positively charged patch is observed around the active site that may be
favourable for substrate binding. The dimer interface has a similar number of
non-covalent interactions as other APs and the "crown"-domain is the
largest observed in known APs. Part of it slopes over the catalytic site
suggesting that the substrates may be small molecules. The catalytic serines are
refined with multiple conformations in both monomers. One of the ligands to the
catalytic zinc ion in binding site M1 is directly connected to the crown-domain
and is closest to the dimer interface. Subtle movements in metal ligands may
help in the release of the product and/or facilitate prior dephosphorylation of
the covalent intermediate. Intersubunit interactions may be a major factor for
promoting active site geometries that lead to the high catalytic activity of
Vibrio AP at low temperatures.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.Koutsioulis,
A.Lyskowski,
S.Mäki,
E.Guthrie,
G.Feller,
V.Bouriotis,
and
P.Heikinheimo
(2010).
Coordination sphere of the third metal site is essential to the activity and metal selectivity of alkaline phosphatases.
|
| |
Protein Sci,
19,
75-84.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
H.Tsuruta,
B.Mikami,
T.Higashi,
and
Y.Aizono
(2010).
Crystal structure of cold-active alkaline phosphatase from the psychrophile Shewanella sp.
|
| |
Biosci Biotechnol Biochem,
74,
69-74.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
X.Mao,
Y.Hong,
Z.Shao,
Y.Zhao,
and
Z.Liu
(2010).
A novel cold-active and alkali-stable β-glucosidase gene isolated from the marine bacterium Martelella mediterranea.
|
| |
Appl Biochem Biotechnol,
162,
2136-2148.
|
 |
|
|
|
|
 |
W.Qiao,
C.Ellis,
J.Steffen,
C.Y.Wu,
and
D.J.Eide
(2009).
Zinc status and vacuolar zinc transporters control alkaline phosphatase accumulation and activity in Saccharomyces cerevisiae.
|
| |
Mol Microbiol,
72,
320-334.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

