 |
PDBsum entry 3cpm

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Plant peptide deformylase pdf1b crystal structure
|
|
Structure:
|
 |
Peptide deformylase, chloroplast. Chain: a. Fragment: residues 65-257. Synonym: pdf, polypeptide deformylase. Engineered: yes
|
|
Source:
|
 |
Arabidopsis thaliana. Mouse-ear cress. Gene: pdf1b. Expressed in: escherichia coli.
|
|
Resolution:
|
 |
|
2.40Å
|
R-factor:
|
0.235
|
R-free:
|
0.297
|
|
|
Authors:
|
 |
D.W.Rodgers,R.L.Houtz,L.M.A.Dirk,J.J.Schmidt,Y.Cai
|
|
Key ref:
|
 |
L.M.Dirk
et al.
(2008).
Insights into the substrate specificity of plant peptide deformylase, an essential enzyme with potential for the development of novel biotechnology applications in agriculture.
Biochem J,
413,
417-427.
PubMed id:
|
 |
|
Date:
|
 |
|
31-Mar-08
|
Release date:
|
22-Jul-08
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9FUZ2
(DEF1B_ARATH) -
Peptide deformylase 1B, chloroplastic/mitochondrial from Arabidopsis thaliana
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
273 a.a.
184 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.1.88
- peptide deformylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-terminal N-formyl-L-methionyl-[peptide] + H2O = N-terminal L-methionyl- [peptide] + formate
|
 |
 |
 |
 |
 |
N-terminal N-formyl-L-methionyl-[peptide]
|
+
|
H2O
|
=
|
N-terminal L-methionyl- [peptide]
|
+
|
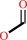 formate
formate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Fe(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochem J
413:417-427
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Insights into the substrate specificity of plant peptide deformylase, an essential enzyme with potential for the development of novel biotechnology applications in agriculture.
|
|
L.M.Dirk,
J.J.Schmidt,
Y.Cai,
J.C.Barnes,
K.M.Hanger,
N.R.Nayak,
M.A.Williams,
R.B.Grossman,
R.L.Houtz,
D.W.Rodgers.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The crystal structure of AtPDF1B [Arabidopsis thaliana PDF (peptide deformylase)
1B; EC 3.5.1.88], a plant specific deformylase, has been determined at a
resolution of 2.4 A (1 A=0.1 nm). The overall fold of AtPDF1B is similar to
other peptide deformylases that have been reported. Evidence from the crystal
structure and gel filtration chromatography indicates that AtPDF1B exists as a
symmetric dimer. PDF1B is essential in plants and has a preferred substrate
specificity towards the PS II (photosystem II) D1 polypeptide. Comparative
analysis of AtPDF1B, AtPDF1A, and the type 1B deformylase from Escherichia coli,
identifies a number of differences in substrate binding subsites that might
account for variations in sequence preference. A model of the N-terminal five
amino acids from the D1 polypeptide bound in the active site of AtPDF1B suggests
an influence of Tyr(178) as a structural determinant for polypeptide substrate
specificity through hydrogen bonding with Thr(2) in the D1 sequence. Kinetic
analyses using a polypeptide mimic of the D1 N-terminus was performed on AtPDF1B
mutated at Tyr(178) to alanine, phenylalanine or arginine (equivalent residue in
AtPDF1A). The results suggest that, whereas Tyr(178) can influence catalytic
activity, other residues contribute to the overall preference for the D1
polypeptide.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Fernández-San Millán,
P.Obregón,
and
J.Veramendi
(2011).
Over-expression of peptide deformylase in chloroplasts confers actinonin resistance, but is not a suitable selective marker system for plastid transformation.
|
| |
Transgenic Res,
20,
613-624.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

