
|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Crystal structure of catalytic domain of human histone deacetylase hdac7 in complex with saha
|
|
Structure:
|
 |
Histone deacetylase 7a. Chain: a, b, c. Fragment: catalytic domain: residues 482-903. Synonym: hd7a, hdac7. Engineered: yes
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: hdac7a, hdac7. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.10Å
|
R-factor:
|
0.199
|
R-free:
|
0.243
|
|
|
Authors:
|
 |
J.Min,A.Schuetz,P.Loppnau,J.Weigelt,M.Sundstrom,C.H.Arrowsmith, A.M.Edwards,A.Bochkarev,A.N.Plotnikov,Structural Genomics Consortium (Sgc)
|
Key ref:

|
 |
A.Schuetz
et al.
(2008).
Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity.
J Biol Chem,
283,
11355-11363.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
21-Jan-08
|
Release date:
|
19-Feb-08
|
|
|
Supersedes:
|

|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
Chains A, B, C:
E.C.3.5.1.98
- histone deacetylase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N6-acetyl-L-lysyl-[histone] + H2O = L-lysyl-[histone] + acetate
|
 |
 |
 |
 |
 |
N(6)-acetyl-L-lysyl-[histone]
|
+
|
H2O
|
=
|
L-lysyl-[histone]
|
+
|
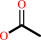 acetate
acetate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
283:11355-11363
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Human HDAC7 harbors a class IIa histone deacetylase-specific zinc binding motif and cryptic deacetylase activity.
|
|
A.Schuetz,
J.Min,
A.Allali-Hassani,
M.Schapira,
M.Shuen,
P.Loppnau,
R.Mazitschek,
N.P.Kwiatkowski,
T.A.Lewis,
R.L.Maglathin,
T.H.McLean,
A.Bochkarev,
A.N.Plotnikov,
M.Vedadi,
C.H.Arrowsmith.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Histone deacetylases (HDACs) are protein deacetylases that play a role in
repression of gene transcription and are emerging targets in cancer therapy.
Here, we characterize the structure and enzymatic activity of the catalytic
domain of human HDAC7 (cdHDAC7). Although HDAC7 normally exists as part of a
multiprotein complex, we show that cdHDAC7 has a low level of deacetylase
activity which can be inhibited by known HDAC inhibitors. The crystal structures
of human cdHDAC7 and its complexes with two hydroxamate inhibitors are the first
structures of the catalytic domain of class IIa HDACs and demonstrate
significant differences with previously reported class I and class IIb-like HDAC
structures. We show that cdHDAC7 has an additional class IIa HDAC-specific zinc
binding motif adjacent to the active site which is likely to participate in
substrate recognition and protein-protein interaction and may provide a site for
modulation of activity. Furthermore, a different active site topology results in
modified catalytic properties and in an enlarged active site pocket. Our studies
provide mechanistic insights into class IIa HDACs and facilitate the design of
specific modulators.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Stereo view of the cdHDAC7 active site. Shown are the
interactions in the structure of apo-cdHDAC7 (A) and in the
cdHDAC7·TSA complex structure (B). Water molecules are
shown as blue spheres and potential hydrogen bonds as blue
dashed lines.
|
 |
Figure 4.
Hydroxamate inhibitor binding of cdHDAC7. A and B, the 2|F[0]
- F[c]| electron density maps for the bound zinc and inhibitor
molecules in the active site of cdHDAC7 are contoured at 0.9σ
(blue). TSA (A) and SAHA (B) are shown as stick models colored
as per atom type: carbon in green, oxygen in red, and nitrogen
in blue. C, the TSA molecule bound in the cdHDAC7 active site is
shown as a sphere model. The histidine residue His-843 points
away from the active site, resulting in an enlarged active site
pocket.
|
 |
|
|

|
| |
The above figures are
reprinted
from an Open Access publication published by the ASBMB:
J Biol Chem
(2008,
283,
11355-11363)
copyright 2008.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
C.H.Arrowsmith,
C.Bountra,
P.V.Fish,
K.Lee,
and
M.Schapira
(2012).
Epigenetic protein families: a new frontier for drug discovery.
|
| |
Nat Rev Drug Discov,
11,
384-400.
|
 |
|
|
|
|
 |
A.Linares,
F.Dalenc,
P.Balaguer,
N.Boulle,
and
V.Cavailles
(2011).
Manipulating protein acetylation in breast cancer: a promising approach in combination with hormonal therapies?
|
| |
J Biomed Biotechnol,
2011,
856985.
|
 |
|
|
|
|
 |
A.Krivoruchko,
and
K.B.Storey
(2010).
Epigenetics in anoxia tolerance: a role for histone deacetylases.
|
| |
Mol Cell Biochem,
342,
151-161.
|
 |
|
|
|
|
 |
J.E.Bradner,
N.West,
M.L.Grachan,
E.F.Greenberg,
S.J.Haggarty,
T.Warnow,
and
R.Mazitschek
(2010).
Chemical phylogenetics of histone deacetylases.
|
| |
Nat Chem Biol,
6,
238-243.
|
 |
|
|
|
|
 |
K.T.Smith,
S.A.Martin-Brown,
L.Florens,
M.P.Washburn,
and
J.L.Workman
(2010).
Deacetylase inhibitors dissociate the histone-targeting ING2 subunit from the Sin3 complex.
|
| |
Chem Biol,
17,
65-74.
|
 |
|
|
|
|
 |
M.Comin,
and
D.Verzotto
(2010).
Classification of protein sequences by means of irredundant patterns.
|
| |
BMC Bioinformatics,
11,
S16.
|
 |
|
|
|
|
 |
R.Higashiyama,
S.Miyaki,
S.Yamashita,
T.Yoshitaka,
G.Lindman,
Y.Ito,
T.Sasho,
K.Takahashi,
M.Lotz,
and
H.Asahara
(2010).
Correlation between MMP-13 and HDAC7 expression in human knee osteoarthritis.
|
| |
Mod Rheumatol,
20,
11-17.
|
 |
|
|
|
|
 |
S.L.Gantt,
C.G.Joseph,
and
C.A.Fierke
(2010).
Activation and inhibition of histone deacetylase 8 by monovalent cations.
|
| |
J Biol Chem,
285,
6036-6043.
|
 |
|
|
|
|
 |
S.Malik,
S.Jiang,
J.P.Garee,
E.Verdin,
A.V.Lee,
B.W.O'Malley,
M.Zhang,
N.S.Belaguli,
and
S.Oesterreich
(2010).
Histone deacetylase 7 and FoxA1 in estrogen-mediated repression of RPRM.
|
| |
Mol Cell Biol,
30,
399-412.
|
 |
|
|
|
|
 |
U.S.Tambunan,
and
E.K.Wulandari
(2010).
Identification of a better Homo sapiens Class II HDAC inhibitor through binding energy calculations and descriptor analysis.
|
| |
BMC Bioinformatics,
11,
S16.
|
 |
|
|
|
|
 |
W.J.Huang,
C.C.Chen,
S.W.Chao,
S.S.Lee,
F.L.Hsu,
Y.L.Lu,
M.F.Hung,
and
C.I.Chang
(2010).
Synthesis of N-hydroxycinnamides capped with a naturally occurring moiety as inhibitors of histone deacetylase.
|
| |
ChemMedChem,
5,
598-607.
|
 |
|
|
|
|
 |
A.Bougdour,
D.Maubon,
P.Baldacci,
P.Ortet,
O.Bastien,
A.Bouillon,
J.C.Barale,
H.Pelloux,
R.Ménard,
and
M.A.Hakimi
(2009).
Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites.
|
| |
J Exp Med,
206,
953-966.
|
 |
|
|
|
|
 |
A.K.Oyelere,
P.C.Chen,
W.Guerrant,
S.C.Mwakwari,
R.Hood,
Y.Zhang,
and
Y.Fan
(2009).
Non-peptide macrocyclic histone deacetylase inhibitors.
|
| |
J Med Chem,
52,
456-468.
|
 |
|
|
|
|
 |
A.Montero,
J.M.Beierle,
C.A.Olsen,
and
M.R.Ghadiri
(2009).
Design, synthesis, biological evaluation, and structural characterization of potent histone deacetylase inhibitors based on cyclic alpha/beta-tetrapeptide architectures.
|
| |
J Am Chem Soc,
131,
3033-3041.
|
 |
|
|
|
|
 |
C.A.Olsen,
and
M.R.Ghadiri
(2009).
Discovery of potent and selective histone deacetylase inhibitors via focused combinatorial libraries of cyclic alpha3beta-tetrapeptides.
|
| |
J Med Chem,
52,
7836-7846.
|
 |
|
|
|
|
 |
C.L.Benn,
R.Butler,
L.Mariner,
J.Nixon,
H.Moffitt,
M.Mielcarek,
B.Woodman,
and
G.P.Bates
(2009).
Genetic knock-down of HDAC7 does not ameliorate disease pathogenesis in the R6/2 mouse model of Huntington's disease.
|
| |
PLoS One,
4,
e5747.
|
 |
|
|
|
|
 |
D.Griffith,
M.P.Morgan,
and
C.J.Marmion
(2009).
A novel anti-cancer bifunctional platinum drug candidate with dual DNA binding and histone deacetylase inhibitory activity.
|
| |
Chem Commun (Camb),
(),
6735-6737.
|
 |
|
|
|
|
 |
D.Wang
(2009).
Computational studies on the histone deacetylases and the design of selective histone deacetylase inhibitors.
|
| |
Curr Top Med Chem,
9,
241-256.
|
 |
|
|
|
|
 |
K.T.Smith,
and
J.L.Workman
(2009).
Histone deacetylase inhibitors: anticancer compounds.
|
| |
Int J Biochem Cell Biol,
41,
21-25.
|
 |
|
|
|
|
 |
L.Wang,
E.F.de Zoeten,
M.I.Greene,
and
W.W.Hancock
(2009).
Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells.
|
| |
Nat Rev Drug Discov,
8,
969-981.
|
 |
|
|
|
|
 |
M.Haberland,
R.L.Montgomery,
and
E.N.Olson
(2009).
The many roles of histone deacetylases in development and physiology: implications for disease and therapy.
|
| |
Nat Rev Genet,
10,
32-42.
|
 |
|
|
|
|
 |
R.Codd,
N.Braich,
J.Liu,
C.Z.Soe,
and
A.A.Pakchung
(2009).
Zn(II)-dependent histone deacetylase inhibitors: suberoylanilide hydroxamic acid and trichostatin A.
|
| |
Int J Biochem Cell Biol,
41,
736-739.
|
 |
|
|
|
|
 |
S.Schäfer,
L.Saunders,
S.Schlimme,
V.Valkov,
J.M.Wagner,
F.Kratz,
W.Sippl,
E.Verdin,
and
M.Jung
(2009).
Pyridylalanine-containing hydroxamic acids as selective HDAC6 inhibitors.
|
| |
ChemMedChem,
4,
283-290.
|
 |
|
|
|
|
 |
T.Suzuki
(2009).
Explorative study on isoform-selective histone deacetylase inhibitors.
|
| |
Chem Pharm Bull (Tokyo),
57,
897-906.
|
 |
|
|
|
|
 |
C.Gao,
C.C.Ho,
E.Reineke,
M.Lam,
X.Cheng,
K.J.Stanya,
Y.Liu,
S.Chakraborty,
H.M.Shih,
and
H.Y.Kao
(2008).
Histone deacetylase 7 promotes PML sumoylation and is essential for PML nuclear body formation.
|
| |
Mol Cell Biol,
28,
5658-5667.
|
 |
|
|
|
|
 |
D.R.Walkinshaw,
S.Tahmasebi,
N.R.Bertos,
and
X.J.Yang
(2008).
Histone deacetylases as transducers and targets of nuclear signaling.
|
| |
J Cell Biochem,
104,
1541-1552.
|
 |
|
|
|
|
 |
D.R.Walkinshaw,
and
X.J.Yang
(2008).
Histone deacetylase inhibitors as novel anticancer therapeutics.
|
| |
Curr Oncol,
15,
237-243.
|
 |
|
|
|
|
 |
P.A.Cole
(2008).
Chemical probes for histone-modifying enzymes.
|
| |
Nat Chem Biol,
4,
590-597.
|
 |
|
|
|
|
 |
Z.Zhou,
X.Song,
B.Li,
and
M.I.Greene
(2008).
FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity.
|
| |
Immunol Res,
42,
19-28.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

