 |
PDBsum entry 3a1i

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.1.4
- amidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a monocarboxylic acid amide + H2O = a monocarboxylate + NH4+
|
 |
 |
 |
 |
 |
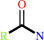 monocarboxylic acid amide
monocarboxylic acid amide
|
+
|
H2O
|
=
|
 monocarboxylate
monocarboxylate
|
+
|
NH4(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochim Biophys Acta
1804:184-192
(2010)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure and characterization of amidase from Rhodococcus sp. N-771: Insight into the molecular mechanism of substrate recognition.
|
|
A.Ohtaki,
K.Murata,
Y.Sato,
K.Noguchi,
H.Miyatake,
N.Dohmae,
K.Yamada,
M.Yohda,
M.Odaka.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
In this study, we have structurally characterized the amidase of a
nitrile-degrading bacterium, Rhodococcus sp. N-771 (RhAmidase). RhAmidase
belongs to amidase signature (AS) family, a group of amidase families, and is
responsible for the degradation of amides produced from nitriles by nitrile
hydratase. Recombinant RhAmidase exists as a dimer of about 107 kDa. RhAmidase
can hydrolyze acetamide, propionamide, acrylamide and benzamide with kcat/Km
values of 1.14+/-0.23 mM(-1)s(-1), 4.54+/-0.09 mM(-1)s(-1), 0.087+/-0.02
mM(-1)s(-1) and 153.5+/-7.1 mM(-1)s(-1), respectively. The crystal structures of
RhAmidase and its inactive mutant complex with benzamide (S195A/benzamide) were
determined at resolutions of 2.17 A and 2.32 A, respectively. RhAmidase has
three domains: an N-terminal alpha-helical domain, a small domain and a large
domain. The N-terminal alpha-helical domain is not found in other AS family
enzymes. This domain is involved in the formation of the dimer structure and,
together with the small domain, forms a narrow substrate-binding tunnel. The
large domain showed high structural similarities to those of other AS family
enzymes. The Ser-cis Ser-Lys catalytic triad is located in the large domain. But
the substrate-binding pocket of RhAmidase is relatively narrow, due to the
presence of the helix alpha13 in the small domain. The hydrophobic residues from
the small domain are involved in recognizing the substrate. The small domain
likely participates in substrate recognition and is related to the difference of
substrate specificities among the AS family amidases.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

