 |
PDBsum entry 2xlc
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Hydrolase
|
 |
|
Title:
|
 |
Acetyl xylan esterase from bacillus pumilus cect5072 bound to paraoxon
|
|
Structure:
|
 |
Acetyl xylan esterase. Chain: a, b, c, d, e, f. Synonym: cephalosporin c deacetylase. Engineered: yes
|
|
Source:
|
 |
Bacillus pumilus. Organism_taxid: 1408. Strain: cect5072. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
2.70Å
|
R-factor:
|
0.217
|
R-free:
|
0.236
|
|
|
Authors:
|
 |
F.Gil-Ortiz,S.Montoro-Garcia,L.M.Polo,V.Rubio,A.Sanchez-Ferrer
|
|
Key ref:
|
 |
S.Montoro-García
et al.
(2011).
The crystal structure of the cephalosporin deacetylating enzyme acetyl xylan esterase bound to paraoxon explains the low sensitivity of this serine hydrolase to organophosphate inactivation.
Biochem J,
436,
321-330.
PubMed id:
|
 |
|
Date:
|
 |
|
20-Jul-10
|
Release date:
|
25-May-11
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q9K5F2
(Q9K5F2_BACPU) -
Acetyl xylan esterase from Bacillus pumilus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
320 a.a.
311 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 6 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.1.6
- acetylesterase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
an acetyl ester + H2O = an aliphatic alcohol + acetate + H+
|
 |
 |
 |
 |
 |
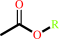 acetyl ester
acetyl ester
|
+
|
H2O
|
=
|
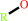 aliphatic alcohol
aliphatic alcohol
|
+
|
 acetate
acetate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochem J
436:321-330
(2011)
|
|
PubMed id:
|
|
|
|

|
| |
|
The crystal structure of the cephalosporin deacetylating enzyme acetyl xylan esterase bound to paraoxon explains the low sensitivity of this serine hydrolase to organophosphate inactivation.
|
|
S.Montoro-García,
F.Gil-Ortiz,
F.García-Carmona,
L.M.Polo,
V.Rubio,
�.�.Sánchez-Ferrer.
|
|
|

|
| |
ABSTRACT
|
|

|
| |
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

