 |
PDBsum entry 2vu2

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Transferase
|
 |
|
Title:
|
 |
Biosynthetic thiolase from z. Ramigera. Complex with s-pantetheine-11- pivalate.
|
|
Structure:
|
 |
Acetyl-coa acetyltransferase. Chain: a, b, c, d. Fragment: residues 2-392. Synonym: acetoacetyl-coa thiolase, biosynthetic thiolase. Engineered: yes
|
|
Source:
|
 |
Zoogloea ramigera. Organism_taxid: 350. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
2.65Å
|
R-factor:
|
0.233
|
R-free:
|
0.286
|
|
|
Authors:
|
 |
P.Kursula,G.Merilainen,W.Schmitz,R.K.Wierenga
|
|
Key ref:
|
 |
G.Meriläinen
et al.
(2008).
The sulfur atoms of the substrate CoA and the catalytic cysteine are required for a productive mode of substrate binding in bacterial biosynthetic thiolase, a thioester-dependent enzyme.
Febs J,
275,
6136-6148.
PubMed id:
|
 |
|
Date:
|
 |
|
19-May-08
|
Release date:
|
28-Oct-08
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P07097
(THIL_SHIZO) -
Acetyl-CoA acetyltransferase from Shinella zoogloeoides
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
392 a.a.
389 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.2.3.1.9
- acetyl-CoA C-acetyltransferase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Mevalonate Biosynthesis
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 acetyl-CoA = acetoacetyl-CoA + CoA
|
 |
 |
 |
 |
 |
 2
×
acetyl-CoA
2
×
acetyl-CoA
|
=
|
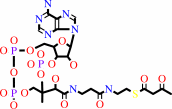 acetoacetyl-CoA
acetoacetyl-CoA
|
+
|
 CoA
CoA
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Febs J
275:6136-6148
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
The sulfur atoms of the substrate CoA and the catalytic cysteine are required for a productive mode of substrate binding in bacterial biosynthetic thiolase, a thioester-dependent enzyme.
|
|
G.Meriläinen,
W.Schmitz,
R.K.Wierenga,
P.Kursula.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Thioesters are more reactive than oxoesters, and thioester chemistry is
important for the reaction mechanisms of many enzymes, including the members of
the thiolase superfamily, which play roles in both degradative and biosynthetic
pathways. In the reaction mechanism of the biosynthetic thiolase, the thioester
moieties of acetyl-CoA and the acetylated catalytic cysteine react with each
other, forming the product acetoacetyl-CoA. Although a number of studies have
been carried out to elucidate the thiolase reaction mechanism at the atomic
level, relatively little is known about the factors determining the affinity of
thiolases towards their substrates. We have carried out crystallographic studies
on the biosynthetic thiolase from Zoogloea ramigera complexed with CoA and three
of its synthetic analogues to compare the binding modes of these related
compounds. The results show that both the CoA terminal SH group and the side
chain SH group of the catalytic Cys89 are crucial for the correct positioning of
substrate in the thiolase catalytic pocket. Furthermore, calorimetric assays
indicate that the mutation of Cys89 into an alanine significantly decreases the
affinity of thiolase towards CoA. Thus, although the sulfur atom of the
thioester moiety is important for the reaction mechanism of thioester-dependent
enzymes, its specific properties can also affect the affinity and competent mode
of binding of the thioester substrates to these enzymes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

