 |
PDBsum entry 2pwg

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.4.99.11
- isomaltulose synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
sucrose = 6-O-alpha-D-glucopyranosyl-D-fructose
|
 |
 |
 |
 |
 |
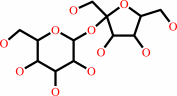 sucrose
sucrose
|
=
|
6-O-alpha-D-glucopyranosyl-D-fructose
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Biol Chem
282:28126-28136
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Trehalulose synthase native and carbohydrate complexed structures provide insights into sucrose isomerization.
|
|
S.Ravaud,
X.Robert,
H.Watzlawick,
R.Haser,
R.Mattes,
N.Aghajari.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Various diseases related to the overconsumption of sugar make a growing need for
sugar substitutes. Because sucrose is an inexpensive and readily available
d-glucose donor, the industrial potential for enzymatic synthesis of the sucrose
isomers trehalulose and/or isomaltulose from sucrose is large. The product
specificity of sucrose isomerases that catalyze this reaction depends
essentially on the possibility for tautomerization of sucrose, which is required
for trehalulose formation. For optimal use of the enzyme, targeting controlled
synthesis of these functional isomers, it is necessary to minimize the side
reactions. This requires an extensive analysis of substrate binding modes and of
the specificity-determining sites in the structure. The 1.6-2.2-A resolution
three-dimensional structures of native and mutant complexes of a trehalulose
synthase from Pseudomonas mesoacidophila MX-45 mimic successive states of the
enzyme reaction. Combined with mutagenesis studies they give for the first time
thorough insights into substrate recognition and processing and reaction
specificities of these enzymes. Among the important outcomes of this study is
the revelation of an aromatic clamp defined by Phe(256) and Phe(280) playing an
essential role in substrate recognition and in controlling the reaction
specificity, which is further supported by mutagenesis studies. Furthermore,
this study highlights essential residues for binding the glucosyl and fructosyl
moieties. The introduction of subtle changes informed by comparative
three-dimensional structural data observed within our study can lead to
fundamental modifications in the mode of action of sucrose isomerases and hence
provide a template for industrial catalysts.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
FIGURE 1. Schematic drawings of the products trehalulose
(A) and isomaltulose (B) and of the substrate sucrose (C).
|
 |
Figure 5.
FIGURE 5. Close up on the active sites of native MutB (A),
E254Q·sucrose (B), MutB·castanospermine (C),
MutB·deoxynojirimycin (D), and D200A·glucose (E).
Electron density 2F[o] - F[c] maps are contoured at 1  .
Catalytic residues are highlighted in green, and other active
site residues are in yellow. The aromatic clamp in magenta
corresponds to conformation 1, and that in cyan corresponds to
conformation 2. .
Catalytic residues are highlighted in green, and other active
site residues are in yellow. The aromatic clamp in magenta
corresponds to conformation 1, and that in cyan corresponds to
conformation 2.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from the ASBMB:
J Biol Chem
(2007,
282,
28126-28136)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
S.Li,
H.Cai,
Y.Qing,
B.Ren,
H.Xu,
H.Zhu,
and
J.Yao
(2011).
Cloning and characterization of a sucrose isomerase from Erwinia rhapontici NX-5 for isomaltulose hyperproduction.
|
| |
Appl Biochem Biotechnol,
163,
52-63.
|
 |
|
|
|
|
 |
W.M.Patrick,
Y.Nakatani,
S.M.Cutfield,
M.L.Sharpe,
R.J.Ramsay,
and
J.F.Cutfield
(2010).
Carbohydrate binding sites in Candida albicans exo-β-1,3-glucanase and the role of the Phe-Phe 'clamp' at the active site entrance.
|
| |
FEBS J,
277,
4549-4561.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
E.Champion,
M.Remaud-Simeon,
L.K.Skov,
J.S.Kastrup,
M.Gajhede,
and
O.Mirza
(2009).
The apo structure of sucrose hydrolase from Xanthomonas campestris pv. campestris shows an open active-site groove.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
1309-1314.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Watzlawick,
and
R.Mattes
(2009).
Gene cloning, protein characterization, and alteration of product selectivity for the trehalulose hydrolase and trehalulose synthase from "Pseudomonas mesoacidophila" MX-45.
|
| |
Appl Environ Microbiol,
75,
7026-7036.
|
 |
|
|
|
|
 |
J.Cha,
J.H.Jung,
S.E.Park,
M.H.Cho,
D.H.Seo,
S.J.Ha,
J.W.Yoon,
O.H.Lee,
Y.C.Kim,
and
C.S.Park
(2009).
Molecular cloning and functional characterization of a sucrose isomerase (isomaltulose synthase) gene from Enterobacter sp. FMB-1.
|
| |
J Appl Microbiol,
107,
1119-1130.
|
 |
|
|
|
|
 |
A.Godány,
B.Vidová,
and
S.Janecek
(2008).
The unique glycoside hydrolase family 77 amylomaltase from Borrelia burgdorferi with only catalytic triad conserved.
|
| |
FEMS Microbiol Lett,
284,
84-91.
|
 |
|
|
|
|
 |
T.Shirai,
V.S.Hung,
K.Morinaka,
T.Kobayashi,
and
S.Ito
(2008).
Crystal structure of GH13 alpha-glucosidase GSJ from one of the deepest sea bacteria.
|
| |
Proteins,
73,
126-133.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

