 |
PDBsum entry 2ptr

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.3.2.2
- adenylosuccinate lyase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Purine Biosynthesis (late stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
|
1.
|
N6-(1,2-dicarboxyethyl)-AMP = fumarate + AMP
|
|
2.
|
(2S)-2-[5-amino-1-(5-phospho-beta-D-ribosyl)imidazole-4- carboxamido]succinate = 5-amino-1-(5-phospho-beta-D-ribosyl)imidazole-4- carboxamide + fumarate
|
|
 |
 |
 |
 |
 |
N(6)-(1,2-dicarboxyethyl)-AMP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
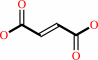 fumarate
fumarate
|
+
|
 AMP
AMP
|
|
 |
 |
 |
 |
 |
(2S)-2-[5-amino-1-(5-phospho-beta-D-ribosyl)imidazole-4- carboxamido]succinate
|
=
|
5-amino-1-(5-phospho-beta-D-ribosyl)imidazole-4- carboxamide
|
+
|
 fumarate
fumarate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Mol Biol
370:541-554
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Substrate and product complexes of Escherichia coli adenylosuccinate lyase provide new insights into the enzymatic mechanism.
|
|
M.Tsai,
J.Koo,
P.Yip,
R.F.Colman,
M.L.Segall,
P.L.Howell.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Adenylosuccinate lyase (ADL) catalyzes the breakdown of 5-aminoimidazole-
(N-succinylocarboxamide) ribotide (SAICAR) to 5-aminoimidazole-4-carboxamide
ribotide (AICAR) and fumarate, and of adenylosuccinate (ADS) to adenosine
monophosphate (AMP) and fumarate in the de novo purine biosynthetic pathway. ADL
belongs to the argininosuccinate lyase (ASL)/fumarase C superfamily of enzymes.
Members of this family share several common features including: a mainly
alpha-helical, homotetrameric structure; three regions of highly conserved amino
acid residues; and a general acid-base catalytic mechanism with the overall
beta-elimination of fumarate as a product. The crystal structures of wild-type
Escherichia coli ADL (ec-ADL), and mutant-substrate (H171A-ADS) and -product
(H171N-AMP.FUM) complexes have been determined to 2.0, 1.85, and 2.0 A
resolution, respectively. The H171A-ADS and H171N-AMP.FUM structures provide the
first detailed picture of the ADL active site, and have enabled the precise
identification of substrate binding and putative catalytic residues. Contrary to
previous suggestions, the ec-ADL structures implicate S295 and H171 in base and
acid catalysis, respectively. Furthermore, structural alignments of ec-ADL with
other superfamily members suggest for the first time a large conformational
movement of the flexible C3 loop (residues 287-303) in ec-ADL upon substrate
binding and catalysis, resulting in its closure over the active site. This loop
movement has been observed in other superfamily enzymes, and has been proposed
to be essential for catalysis. The ADL catalytic mechanism is re-examined in
light of the results presented here.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Stereo view of the superimposed H171A (pink) and
H171N (green) active sites, showing the interactions involving
the AMP (a) and fumarate groups (c). The σ[A] weighted
F[o]–F[c] omit maps for the substrate (orange) and products
(blue) in the H171A and H171N proteins, respectively, are shown
contoured at 3σ. Water molecules are shown as spheres. The
corresponding schematic representations for the AMP and fumarate
groups are shown in (b) and (d), respectively. Hydrogen bonds
are represented as red broken lines with the distances indicated
in angstroms (Å). Distances for the H171N protein are in
bold, and the distances for active site 2 of each protein are
given in parentheses. The letter following the residue number
denotes the monomer to which each residue belongs, with those
for active site 2 of the proteins shown in parentheses. In (b),
residues involved in coordinating water molecules are colored
blue. Poor electron density did not allow residues S295 and S296
in active site 2 of the H171A protein to be modeled. PyMol was
used for Figure preparation.
|
 |
Figure 3.
Figure 3. Stereo view of the superimposed H171A–ADS (pink),
H171N–AMP•FUM (blue) and dδc1-SO[4]^2- (yellow) active
sites, showing the conformation of the C3 loop in the proteins.
Since the C3 loop in the SeMet protein could not be modeled due
to the absence of electron density, the open conformation of the
loop observed in the dδc2-S281A mutant^12 is also shown for
comparison (green). The side-chains of selected C3 loop residues
are shown and numbered according to the E. coli ADL sequence.
PyMol was used for Figure preparation.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Elsevier:
J Mol Biol
(2007,
370,
541-554)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
G.Allegri,
M.J.Fernandes,
F.B.Scalco,
P.Correia,
R.E.Simoni,
J.C.Llerena,
and
M.L.de Oliveira
(2010).
Fumaric aciduria: an overview and the first Brazilian case report.
|
| |
J Inherit Metab Dis,
33,
411-419.
|
 |
|
|
|
|
 |
P.K.Fyfe,
A.Dawson,
M.T.Hutchison,
S.Cameron,
and
W.N.Hunter
(2010).
Structure of Staphylococcus aureus adenylosuccinate lyase (PurB) and assessment of its potential as a target for structure-based inhibitor discovery.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
881-888.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
A.J.Knox,
C.Graham,
J.Bleskan,
G.Brodsky,
and
D.Patterson
(2009).
Mutations in the Chinese hamster ovary cell GART gene of de novo purine synthesis.
|
| |
Gene,
429,
23-30.
|
 |
|
|
|
|
 |
G.Kozlov,
L.Nguyen,
J.Pearsall,
and
K.Gehring
(2009).
The structure of phosphate-bound Escherichia coli adenylosuccinate lyase identifies His171 as a catalytic acid.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
857-861.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
V.Puthan Veetil,
H.Raj,
W.J.Quax,
D.B.Janssen,
and
G.J.Poelarends
(2009).
Site-directed mutagenesis, kinetic and inhibition studies of aspartate ammonia lyase from Bacillus sp. YM55-1.
|
| |
FEBS J,
276,
2994-3007.
|
 |
|
|
|
|
 |
S.Sivendran,
and
R.F.Colman
(2008).
Effect of a new non-cleavable substrate analog on wild-type and serine mutants in the signature sequence of adenylosuccinate lyase of Bacillus subtilis and Homo sapiens.
|
| |
Protein Sci,
17,
1162-1174.
|
 |
|
|
|
|
 |
Y.Zhang,
M.Morar,
and
S.E.Ealick
(2008).
Structural biology of the purine biosynthetic pathway.
|
| |
Cell Mol Life Sci,
65,
3699-3724.
|
 |
|
|
|
|
 |
S.Sivendran,
M.L.Segall,
P.C.Rancy,
and
R.F.Colman
(2007).
Effect of Asp69 and Arg310 on the pK of His68, a key catalytic residue of adenylosuccinate lyase.
|
| |
Protein Sci,
16,
1700-1707.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

