 |
PDBsum entry 2msd

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Lipid binding protein
|
PDB id
|
|

|

|
2msd
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lipid binding protein
|
 |
|
Title:
|
 |
Nmr data-driven model of gtpase kras-gnp tethered to a lipid-bilayer nanodisc
|
|
Structure:
|
 |
Apolipoprotein a-i. Chain: a, c. Fragment: unp residues 68-265. Synonym: apoa-i,apolipoprotein a1. Engineered: yes. Gtpase kras. Chain: b. Fragment: unp residues 1-185. Synonym: k-ras 2,ki-ras,c-k-ras,c-ki-ras.
|
|
Source:
|
 |
Homo sapiens. Human. Organism_taxid: 9606. Gene: apoa1. Expressed in: escherichia coli. Expression_system_taxid: 562. Gene: kras, kras2, rask2.
|
|
NMR struc:
|
 |
10 models
|
 |
|
Authors:
|
 |
M.Mazhab-Jafari,P.Stathopoulos,C.Marshall,M.Ikura
|
|
Key ref:
|
 |
M.T.Mazhab-Jafari
et al.
(2015).
Oncogenic and RASopathy-associated K-RAS mutations relieve membrane-dependent occlusion of the effector-binding site.
Proc Natl Acad Sci U S A,
112,
6625-6630.
PubMed id:
DOI:
|
 |
|
Date:
|
 |
|
29-Jul-14
|
Release date:
|
03-Jun-15
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, C:
E.C.?
|
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chain B:
E.C.3.6.5.2
- small monomeric GTPase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
GTP + H2O = GDP + phosphate + H+
|
 |
 |
 |
 |
 |
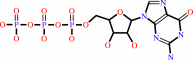 GTP
GTP
|
+
|
H2O
|
=
|
GDP
Bound ligand (Het Group name = )
matches with 81.82% similarity
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
112:6625-6630
(2015)
|
|
PubMed id:
|
|
|
|

|
| |
|
Oncogenic and RASopathy-associated K-RAS mutations relieve membrane-dependent occlusion of the effector-binding site.
|
|
M.T.Mazhab-Jafari,
C.B.Marshall,
M.J.Smith,
G.M.Gasmi-Seabrook,
P.B.Stathopulos,
F.Inagaki,
L.E.Kay,
B.G.Neel,
M.Ikura.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
K-RAS4B (Kirsten rat sarcoma viral oncogene homolog 4B) is a prenylated,
membrane-associated GTPase protein that is a critical switch for the propagation
of growth factor signaling pathways to diverse effector proteins, including
rapidly accelerated fibrosarcoma (RAF) kinases and RAS-related protein guanine
nucleotide dissociation stimulator (RALGDS) proteins. Gain-of-function KRAS
mutations occur frequently in human cancers and predict poor clinical outcome,
whereas germ-line mutations are associated with developmental syndromes.
However, it is not known how these mutations affect K-RAS association with
biological membranes or whether this impacts signal transduction. Here, we used
solution NMR studies of K-RAS4B tethered to nanodiscs to investigate lipid
bilayer-anchored K-RAS4B and its interactions with effector protein RAS-binding
domains (RBDs). Unexpectedly, we found that the effector-binding region of
activated K-RAS4B is occluded by interaction with the membrane in one of the
NMR-observable, and thus highly populated, conformational states. Binding of the
RAF isoform ARAF and RALGDS RBDs induced marked reorientation of K-RAS4B from
the occluded state to RBD-specific effector-bound states. Importantly, we found
that two Noonan syndrome-associated mutations, K5N and D153V, which do not
affect the GTPase cycle, relieve the occluded orientation by directly altering
the electrostatics of two membrane interaction surfaces. Similarly, the most
frequent KRAS oncogenic mutation G12D also drives K-RAS4B toward an exposed
configuration. Further, the D153V and G12D mutations increase the rate of
association of ARAF-RBD with lipid bilayer-tethered K-RAS4B. We revealed a
mechanism of K-RAS4B autoinhibition by membrane sequestration of its
effector-binding site, which can be disrupted by disease-associated mutations.
Stabilizing the autoinhibitory interactions between K-RAS4B and the membrane
could be an attractive target for anticancer drug discovery.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|

