 |
PDBsum entry 2k1g

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lipoprotein
|
 |
|
Title:
|
 |
Solution nmr structure of lipoprotein spr from escherichia coli k12. Northeast structural genomics target er541-37-162
|
|
Structure:
|
 |
Lipoprotein spr. Chain: a. Fragment: residues 63-188. Engineered: yes
|
|
Source:
|
 |
Escherichia coli. Organism_taxid: 83333. Strain: k12. Gene: spr, yeiv, b2175, jw2163. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
NMR struc:
|
 |
20 models
|
 |
|
Authors:
|
 |
J.M.Aramini,P.Rossi,L.Zhao,M.Jiang,M.Maglaqui,R.Xiao,J.Liu,M.C.Baran, G.V.T.Swapna,Y.J.Huang,T.B.Acton,B.Rost,G.T.Montelione,Northeast Structural Genomics Consortium (Nesg)
|
|
Key ref:
|
 |
J.M.Aramini
et al.
(2008).
Solution NMR structure of the NlpC/P60 domain of lipoprotein Spr from Escherichia coli: structural evidence for a novel cysteine peptidase catalytic triad.
Biochemistry,
47,
9715-9717.
PubMed id:
|
 |
|
Date:
|
 |
|
03-Mar-08
|
Release date:
|
18-Mar-08
|
|
|
Supersedes:
|

|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P0AFV4
(MEPS_ECOLI) -
Murein DD-endopeptidase MepS/Murein LD-carboxypeptidase from Escherichia coli (strain K12)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
188 a.a.
129 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 1 residue position (black
cross)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
E.C.3.4.-.-
|
|
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
E.C.3.4.17.13
- muramoyltetrapeptide carboxypeptidase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
N-acetyl-D-glucosaminyl-N-acetylmuramoyl-L-alanyl-meso-2,6- diaminoheptanedioyl-D-alanine + H2O = N-acetyl-D-glucosaminyl-N- acetylmuramoyl-L-alanyl-meso-2,6-diaminoheptanedioate + D-alanine
|
 |
 |
 |
 |
 |
N-acetyl-D-glucosaminyl-N-acetylmuramoyl-L-alanyl-meso-2,6- diaminoheptanedioyl-D-alanine
|
+
|
H2O
|
=
|
N-acetyl-D-glucosaminyl-N- acetylmuramoyl-L-alanyl-meso-2,6-diaminoheptanedioate
|
+
|
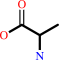 D-alanine
D-alanine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Biochemistry
47:9715-9717
(2008)
|
|
PubMed id:
|
|
|
|

|
| |
|
Solution NMR structure of the NlpC/P60 domain of lipoprotein Spr from Escherichia coli: structural evidence for a novel cysteine peptidase catalytic triad.
|
|
J.M.Aramini,
P.Rossi,
Y.J.Huang,
L.Zhao,
M.Jiang,
M.Maglaqui,
R.Xiao,
J.Locke,
R.Nair,
B.Rost,
T.B.Acton,
M.Inouye,
G.T.Montelione.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Escherichia coli Spr is a membrane-anchored cell wall hydrolase. The solution
NMR structure of the C-terminal NlpC/P60 domain of E. coli Spr described here
reveals that the protein adopts a papain-like alpha+beta fold and identifies a
substrate-binding cleft featuring several highly conserved residues. The active
site features a novel Cys-His-His catalytic triad that appears to be a unique
structural signature of this cysteine peptidase family. Moreover, the relative
orientation of these catalytic residues is similar to that observed in the
analogous Ser-His-His triad, a variant of the classic Ser-His-Asp charge relay
system, suggesting the convergent evolution of a catalytic mechanism in quite
distinct peptidase families.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Ruggiero,
D.Marasco,
F.Squeglia,
S.Soldini,
E.Pedone,
C.Pedone,
and
R.Berisio
(2010).
Structure and functional regulation of RipA, a mycobacterial enzyme essential for daughter cell separation.
|
| |
Structure,
18,
1184-1190.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
Q.Xu,
P.Abdubek,
T.Astakhova,
H.L.Axelrod,
C.Bakolitsa,
X.Cai,
D.Carlton,
C.Chen,
H.J.Chiu,
M.Chiu,
T.Clayton,
D.Das,
M.C.Deller,
L.Duan,
K.Ellrott,
C.L.Farr,
J.Feuerhelm,
J.C.Grant,
A.Grzechnik,
G.W.Han,
L.Jaroszewski,
K.K.Jin,
H.E.Klock,
M.W.Knuth,
P.Kozbial,
S.S.Krishna,
A.Kumar,
W.W.Lam,
D.Marciano,
M.D.Miller,
A.T.Morse,
E.Nigoghossian,
A.Nopakun,
L.Okach,
C.Puckett,
R.Reyes,
H.J.Tien,
C.B.Trame,
H.van den Bedem,
D.Weekes,
T.Wooten,
A.Yeh,
K.O.Hodgson,
J.Wooley,
M.A.Elsliger,
A.M.Deacon,
A.Godzik,
S.A.Lesley,
and
I.A.Wilson
(2010).
Structure of the γ-D-glutamyl-L-diamino acid endopeptidase YkfC from Bacillus cereus in complex with L-Ala-γ-D-Glu: insights into substrate recognition by NlpC/P60 cysteine peptidases.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
66,
1354-1364.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
P.Rossi,
J.M.Aramini,
R.Xiao,
C.X.Chen,
C.Nwosu,
L.A.Owens,
M.Maglaqui,
R.Nair,
M.Fischer,
T.B.Acton,
B.Honig,
B.Rost,
and
G.T.Montelione
(2009).
Structural elucidation of the Cys-His-Glu-Asn proteolytic relay in the secreted CHAP domain enzyme from the human pathogen Staphylococcus saprophyticus.
|
| |
Proteins,
74,
515-519.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

