 |
PDBsum entry 2j5b

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.1
- tyrosine--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Tyr) + L-tyrosine + ATP = L-tyrosyl-tRNA(Tyr) + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
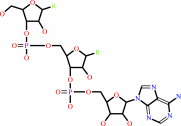 tRNA(Tyr)
tRNA(Tyr)
|
+
|
L-tyrosine
Bound ligand (Het Group name = )
matches with 92.31% similarity
|
+
|
 ATP
ATP
|
=
|
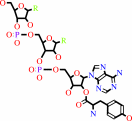 L-tyrosyl-tRNA(Tyr)
L-tyrosyl-tRNA(Tyr)
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
J Virol
81:12406-12417
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Virus-encoded aminoacyl-tRNA synthetases: structural and functional characterization of mimivirus TyrRS and MetRS.
|
|
C.Abergel,
J.Rudinger-Thirion,
R.Giegé,
J.M.Claverie.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Aminoacyl-tRNA synthetases are pivotal in determining how the genetic code is
translated in amino acids and in providing the substrate for protein synthesis.
As such, they fulfill a key role in a process universally conserved in all
cellular organisms from their most complex to their most reduced parasitic
forms. In contrast, even complex viruses were not found to encode much
translation machinery, with the exception of isolated components such as tRNAs.
In this context, the discovery of four aminoacyl-tRNA synthetases encoded in the
genome of mimivirus together with a full set of translation initiation,
elongation, and termination factors appeared to blur what was once a clear
frontier between the cellular and viral world. Functional studies of two
mimivirus tRNA synthetases confirmed the MetRS specificity for methionine and
the TyrRS specificity for tyrosine and conformity with the identity rules for
tRNA(Tyr) for archea/eukarya. The atomic structure of the mimivirus tyrosyl-tRNA
synthetase in complex with tyrosinol exhibits the typical fold and active-site
organization of archaeal-type TyrRS. However, the viral enzyme presents a unique
dimeric conformation and significant differences in its anticodon binding site.
The present work suggests that mimivirus aminoacyl-tRNA synthetases function as
regular translation enzymes in infected amoebas. Their phylogenetic
classification does not suggest that they have been acquired recently by
horizontal gene transfer from a cellular host but rather militates in favor of
an intricate evolutionary relationship between large DNA viruses and ancestral
eukaryotes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
M.Legendre,
S.Audic,
O.Poirot,
P.Hingamp,
V.Seltzer,
D.Byrne,
A.Lartigue,
M.Lescot,
A.Bernadac,
J.Poulain,
C.Abergel,
and
J.M.Claverie
(2010).
mRNA deep sequencing reveals 75 new genes and a complex transcriptional landscape in Mimivirus.
|
| |
Genome Res,
20,
664-674.
|
 |
|
|
|
|
 |
R.W.Smith,
and
N.K.Gray
(2010).
Poly(A)-binding protein (PABP): a common viral target.
|
| |
Biochem J,
426,
1.
|
 |
|
|
|
|
 |
D.Byrne,
R.Grzela,
A.Lartigue,
S.Audic,
S.Chenivesse,
S.Encinas,
J.M.Claverie,
and
C.Abergel
(2009).
The polyadenylation site of Mimivirus transcripts obeys a stringent 'hairpin rule'.
|
| |
Genome Res,
19,
1233-1242.
|
 |
|
|
|
|
 |
J.M.Claverie,
and
C.Abergel
(2009).
Mimivirus and its virophage.
|
| |
Annu Rev Genet,
43,
49-66.
|
 |
|
|
|
|
 |
N.Wegner,
R.Wait,
and
P.J.Venables
(2009).
Evolutionarily conserved antigens in autoimmune disease: implications for an infective aetiology.
|
| |
Int J Biochem Cell Biol,
41,
390-397.
|
 |
|
|
|
|
 |
S.Jeudy,
A.Lartigue,
J.M.Claverie,
and
C.Abergel
(2009).
Dissecting the unique nucleotide specificity of mimivirus nucleoside diphosphate kinase.
|
| |
J Virol,
83,
7142-7150.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.Monier,
J.B.Larsen,
R.A.Sandaa,
G.Bratbak,
J.M.Claverie,
and
H.Ogata
(2008).
Marine mimivirus relatives are probably large algal viruses.
|
| |
Virol J,
5,
12.
|
 |
|
|
|
|
 |
D.Benarroch,
P.Smith,
and
S.Shuman
(2008).
Characterization of a trifunctional mimivirus mRNA capping enzyme and crystal structure of the RNA triphosphatase domain.
|
| |
Structure,
16,
501-512.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

