 |
PDBsum entry 2i8c

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Lyase
|
 |
|
Title:
|
 |
Allosteric inhibition of staphylococcus aureus d-alanine:d-alanine ligase revealed by crystallographic studies
|
|
Structure:
|
 |
D-alanine-d-alanine ligase. Chain: a, b. Synonym: d-alanylalanine synthetase, d-ala-d-ala ligase. Engineered: yes
|
|
Source:
|
 |
Staphylococcus aureus subsp. Aureus. Organism_taxid: 93062. Strain: col. Gene: ddl. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Biol. unit:
|
 |
Dimer (from
)
|
|
Resolution:
|
 |
|
2.46Å
|
R-factor:
|
0.213
|
R-free:
|
0.269
|
|
|
Authors:
|
 |
S.Liu,J.S.Chang,J.T.Herberg,M.Horng,P.K.Tomich,A.H.Lin,K.R.Marotti
|
Key ref:

|
 |
S.Liu
et al.
(2006).
Allosteric inhibition of Staphylococcus aureus D-alanine:D-alanine ligase revealed by crystallographic studies.
Proc Natl Acad Sci U S A,
103,
15178-15183.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
01-Sep-06
|
Release date:
|
26-Sep-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
Q5HEB7
(DDL_STAAC) -
D-alanine--D-alanine ligase from Staphylococcus aureus (strain COL)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
356 a.a.
345 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.2.4
- D-alanine--D-alanine ligase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Peptidoglycan Biosynthesis (Part 1)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
2 D-alanine + ATP = D-alanyl-D-alanine + ADP + phosphate + H+
|
 |
 |
 |
 |
 |
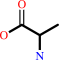 2
×
D-alanine
2
×
D-alanine
|
+
|
 ATP
ATP
|
=
|
D-alanyl-D-alanine
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 ADP
ADP
|
+
|
 phosphate
phosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
103:15178-15183
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Allosteric inhibition of Staphylococcus aureus D-alanine:D-alanine ligase revealed by crystallographic studies.
|
|
S.Liu,
J.S.Chang,
J.T.Herberg,
M.M.Horng,
P.K.Tomich,
A.H.Lin,
K.R.Marotti.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
D-alanine:D-alanine ligase (DDl) is an essential enzyme in bacterial cell wall
biosynthesis and an important target for developing new antibiotics. It
catalyzes the formation of D-alanine:D-alanine dipeptide, sequentially by using
one D-alanine and one ATP as substrates for the first-half reaction, and a
second D-alanine substrate to complete the reaction. Some gain of function DDl
mutants can use an alternate second substrate, causing resistance to vancomycin,
one of the last lines of defense against life-threatening Gram-positive
infections. Here, we report the crystal structure of Staphylococcus aureus DDl
(StaDDl) and its cocrystal structures with
3-chloro-2,2-dimethyl-N-[4(trifluoromethyl)phenyl]propanamide (inhibitor 1)
(Ki=4 microM against StaDDl) and with ADP, one of the reaction products, at
resolutions of 2.0, 2.2, and 2.6 A, respectively. The overall structure of
StaDDl can be divided into three distinct domains. The inhibitor binds to a
hydrophobic pocket at the interface of the first and the third domain. This
inhibitor-binding pocket is adjacent to the first D-alanine substrate site but
does not overlap with any substrate sites. An allosteric inhibition mechanism of
StaDDl by this compound was proposed. The mechanism provides the basis for
developing new antibiotics targeting D-alanine:D-alanine ligase. Because this
compound only interacts with residues from the first D-alanine site, inhibitors
with this binding mode potentially could overcome vancomycin resistance.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Fig. 3. A composite surface representation of StaDDl
ligand-binding sites. The electrostatic protein surface was
constructed from apo StaDDl structure, with red and blue
representing negative and positive charges, respectively, white
is for neutral. Ligands are in stick model. ADP and the first
Mg^2+ (Mg1) are from ADP+Mg^2+-bound StaDDl structure. The
phosphorylated phosphinate and the second Mg^2+ (Mg2) are
modeled in from substrates bound LmDDl2 monomer by
superimposition by using program LSQMAN (20). Inhibitor 1 is
from inhibitor 1–StaDDl complex. For clarity, the carbon atoms
of ADP, phosphinate, and inhibitor 1 are shown in cyan, yellow,
and magenta color, respectively.
|
 |
Figure 5.
Fig. 5. Structure superposition of inhibitor 1-bound StaDDl
with reaction intermediate analog bound LmDDl2. Residues
essential for the first half-reaction are labeled (see text).
For clarity, carbon atoms of StaDDl, inhibitor 1, and
phosphinate are colored cyan, magenta, and white, respectively.
|
 |
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
D.Meziane-Cherif,
F.A.Saul,
C.Moubareck,
P.Weber,
A.Haouz,
P.Courvalin,
and
B.Périchon
(2010).
Molecular basis of vancomycin dependence in VanA-type Staphylococcus aureus VRSA-9.
|
| |
J Bacteriol,
192,
5465-5471.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Moubareck,
D.Meziane-Cherif,
P.Courvalin,
and
B.Périchon
(2009).
VanA-type Staphylococcus aureus strain VRSA-7 is partially dependent on vancomycin for growth.
|
| |
Antimicrob Agents Chemother,
53,
3657-3663.
|
 |
|
|
|
|
 |
K.Kurokawa,
H.Hamamoto,
M.Matsuo,
S.Nishida,
N.Yamane,
B.L.Lee,
K.Murakami,
H.Maki,
and
K.Sekimizu
(2009).
Evaluation of target specificity of antibacterial agents using Staphylococcus aureus ddlA mutants and D-cycloserine in a silkworm infection model.
|
| |
Antimicrob Agents Chemother,
53,
4025-4027.
|
 |
|
|
|
|
 |
S.M.Firestine,
H.Paritala,
J.E.McDonnell,
J.B.Thoden,
and
H.M.Holden
(2009).
Identification of inhibitors of N5-carboxyaminoimidazole ribonucleotide synthetase by high-throughput screening.
|
| |
Bioorg Med Chem,
17,
3317-3323.
|
 |
|
|
|
|
 |
Y.Kitamura,
A.Ebihara,
Y.Agari,
A.Shinkai,
K.Hirotsu,
and
S.Kuramitsu
(2009).
Structure of D-alanine-D-alanine ligase from Thermus thermophilus HB8: cumulative conformational change and enzyme-ligand interactions.
|
| |
Acta Crystallogr D Biol Crystallogr,
65,
1098-1106.
|
 |
|
|
|
|
 |
A.A.Miller,
G.L.Bundy,
J.E.Mott,
J.E.Skepner,
T.P.Boyle,
D.W.Harris,
A.E.Hromockyj,
K.R.Marotti,
G.E.Zurenko,
J.B.Munzner,
M.T.Sweeney,
G.F.Bammert,
J.C.Hamel,
C.W.Ford,
W.Z.Zhong,
D.R.Graber,
G.E.Martin,
F.Han,
L.A.Dolak,
E.P.Seest,
J.C.Ruble,
G.M.Kamilar,
J.R.Palmer,
L.S.Banitt,
A.R.Hurd,
and
M.R.Barbachyn
(2008).
Discovery and characterization of QPT-1, the progenitor of a new class of bacterial topoisomerase inhibitors.
|
| |
Antimicrob Agents Chemother,
52,
2806-2812.
|
 |
|
|
|
|
 |
D.Wu,
L.Zhang,
Y.Kong,
J.Du,
S.Chen,
J.Chen,
J.Ding,
H.Jiang,
and
X.Shen
(2008).
Enzymatic characterization and crystal structure analysis of the D-alanine-D-alanine ligase from Helicobacter pylori.
|
| |
Proteins,
72,
1148-1160.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
H.Barreteau,
A.Kovac,
A.Boniface,
M.Sova,
S.Gobec,
and
D.Blanot
(2008).
Cytoplasmic steps of peptidoglycan biosynthesis.
|
| |
FEMS Microbiol Rev,
32,
168-207.
|
 |
|
|
|
|
 |
T.T.Doan,
J.K.Kim,
H.Kim,
Y.J.Ahn,
J.G.Kim,
B.M.Lee,
and
L.W.Kang
(2008).
Expression, crystallization and preliminary X-ray crystallographic analysis of Xoo0352, D-alanine-D-alanine ligase A, from Xanthomonas oryzae pv. oryzae.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
1115-1117.
|
 |
|
|
|
|
 |
K.Ohlsen,
and
U.Lorenz
(2007).
Novel targets for antibiotics in Staphylococcus aureus.
|
| |
Future Microbiol,
2,
655-666.
|
 |
|
|
|
|
 |
Y.Z.Lu,
Y.Sheng,
L.F.Li,
D.W.Tang,
X.Y.Liu,
X.Zhao,
Y.H.Liang,
and
X.D.Su
(2007).
Crystallization and preliminary crystallographic analysis of D-alanine-D-alanine ligase from Streptococcus mutans.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
63,
807-808.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

