 |
PDBsum entry 2g4j

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Isomerase/metal-binding protein
|
PDB id
|
|

|

|
2g4j
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.5.3.1.5
- xylose isomerase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
alpha-D-xylose = alpha-D-xylulofuranose
|
 |
 |
 |
 |
 |
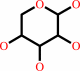 alpha-D-xylose
alpha-D-xylose
|
=
|
alpha-D-xylulofuranose
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Mg(2+)
|
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Acta Crystallogr D Biol Crystallogr
63:366-380
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
On the routine use of soft X-rays in macromolecular crystallography. Part IV. Efficient determination of anomalous substructures in biomacromolecules using longer X-ray wavelengths.
|
|
C.Mueller-Dieckmann,
S.Panjikar,
A.Schmidt,
S.Mueller,
J.Kuper,
A.Geerlof,
M.Wilmanns,
R.K.Singh,
P.A.Tucker,
M.S.Weiss.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
23 different crystal forms of 19 different biological macromolecules were
examined with respect to their anomalously scattering substructures using
diffraction data collected at a wavelength of 2.0 A (6.2 keV). In more than 90%
of the cases the substructure was found to contain more than just the protein S
atoms. The data presented suggest that chloride, sulfate, phosphate or metal
ions from the buffer or even from the purification protocol are frequently bound
to the protein molecule and that these ions are often overlooked, especially if
they are not bound at full occupancy. Thus, in order to fully describe the
macromolecule under study, it seems desirable that any structure determination
be complemented with a long-wavelength data set.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |
Figure 1.
Figure 1 Anomalous scattering length (  f'']
) values in units of electrons at f'']
) values in units of electrons at  =
1.0 Å (red) and =
1.0 Å (red) and  =
2.0 Å (green) for elements 11-20 according to Cromer &
Liberman (1970[Cromer, D. & Liberman, D. (1970). J. Chem. Phys.
53, 1891-1898.]). =
2.0 Å (green) for elements 11-20 according to Cromer &
Liberman (1970[Cromer, D. & Liberman, D. (1970). J. Chem. Phys.
53, 1891-1898.]).
|
 |
|
|

|
| |
The above figure is
reprinted
by permission from the IUCr:
Acta Crystallogr D Biol Crystallogr
(2007,
63,
366-380)
copyright 2007.
|
|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
N.S.Pannu,
W.J.Waterreus,
P.Skubák,
I.Sikharulidze,
J.P.Abrahams,
and
R.A.de Graaff
(2011).
Recent advances in the CRANK software suite for experimental phasing.
|
| |
Acta Crystallogr D Biol Crystallogr,
67,
331-337.
|
 |
|
|
|
|
 |
R.J.Read,
and
A.J.McCoy
(2011).
Using SAD data in Phaser.
|
| |
Acta Crystallogr D Biol Crystallogr,
67,
338-344.
|
 |
|
|
|
|
 |
R.M.Leal,
G.P.Bourenkov,
O.Svensson,
D.Spruce,
M.Guijarro,
and
A.N.Popov
(2011).
Experimental procedure for the characterization of radiation damage in macromolecular crystals.
|
| |
J Synchrotron Radiat,
18,
381-386.
|
 |
|
|
|
|
 |
G.L.Taylor
(2010).
Introduction to phasing.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
325-338.
|
 |
|
|
|
|
 |
J.Gabadinho,
A.Beteva,
M.Guijarro,
V.Rey-Bakaikoa,
D.Spruce,
M.W.Bowler,
S.Brockhauser,
D.Flot,
E.J.Gordon,
D.R.Hall,
B.Lavault,
A.A.McCarthy,
J.McCarthy,
E.Mitchell,
S.Monaco,
C.Mueller-Dieckmann,
D.Nurizzo,
R.B.Ravelli,
X.Thibault,
M.A.Walsh,
G.A.Leonard,
and
S.M.McSweeney
(2010).
MxCuBE: a synchrotron beamline control environment customized for macromolecular crystallography experiments.
|
| |
J Synchrotron Radiat,
17,
700-707.
|
 |
|
|
|
|
 |
P.Skubák,
W.J.Waterreus,
and
N.S.Pannu
(2010).
Multivariate phase combination improves automated crystallographic model building.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
783-788.
|
 |
|
|
|
|
 |
A.Guskov,
J.Kern,
A.Gabdulkhakov,
M.Broser,
A.Zouni,
and
W.Saenger
(2009).
Cyanobacterial photosystem II at 2.9-A resolution and the role of quinones, lipids, channels and chloride.
|
| |
Nat Struct Mol Biol,
16,
334-342.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
B.Z.Wang,
P.Guo,
B.J.Hang,
L.Li,
J.He,
and
S.P.Li
(2009).
Cloning of a novel pyrethroid-hydrolyzing carboxylesterase gene from Sphingobium sp. strain JZ-1 and characterization of the gene product.
|
| |
Appl Environ Microbiol,
75,
5496-5500.
|
 |
|
|
|
|
 |
S.B.Larson,
J.S.Day,
C.Nguyen,
R.Cudney,
and
A.McPherson
(2009).
High-resolution structure of proteinase K cocrystallized with digalacturonic acid.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
65,
192-198.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
C.Mueller-Dieckmann,
S.Kernstock,
J.Mueller-Dieckmann,
M.S.Weiss,
and
F.Koch-Nolte
(2008).
Structure of mouse ADP-ribosylhydrolase 3 (mARH3).
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
64,
156-162.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

