
|

|

|
|
 |
Contents |
 |
|
|

|

|

|

|

|

|

|
 446 a.a.
446 a.a.
|
 |

|

|

|

|

|

|

|
 423 a.a.
423 a.a.
|
 |

|

|

|

|

|

|

|
 378 a.a.
378 a.a.
|
 |

|

|

|

|

|

|

|
 241 a.a.
241 a.a.
|
 |

|

|

|

|

|

|

|
 196 a.a.
196 a.a.
|
 |

|

|

|

|

|

|

|
 106 a.a.
106 a.a.
|
 |

|

|

|

|

|

|

|
 75 a.a.
75 a.a.
|
 |

|

|

|

|

|

|

|
 64 a.a.
64 a.a.
|
 |

|

|

|

|

|

|

|
 57 a.a.
57 a.a.
|
 |

|

|

|

|

|

|

|
 60 a.a.
60 a.a.
|
 |

|

|

|

|

|

|

|
 53 a.a.
53 a.a.
|
 |

|

|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
Crystal structure of bovine heart mitochondrial bc1 with jg144 inhibitor
|
|
Structure:
|
 |
Ubiquinol-cytochromE-C reductase complex core protein i, mitochondrial. Chain: a. Fragment: core1. Ubiquinol-cytochromE-C reductase complex core protein 2, mitochondrial. Chain: b. Fragment: core2. Synonym: complex iii subunit ii.
|
|
Source:
|
 |
Bos taurus. Cattle. Organism_taxid: 9913. Organism_taxid: 9913
|
|
Biol. unit:
|
 |
 22mer (from PDB file)
22mer (from PDB file)
|
|
Resolution:
|
 |
|
2.26Å
|
R-factor:
|
0.249
|
R-free:
|
0.283
|
|
|
Authors:
|
 |
D.Xia,L.Esser
|
Key ref:

|
 |
L.Esser
et al.
(2006).
Surface-modulated motion switch: capture and release of iron-sulfur protein in the cytochrome bc1 complex.
Proc Natl Acad Sci U S A,
103,
13045-13050.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
08-Feb-06
|
Release date:
|
29-Aug-06
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P31800
(QCR1_BOVIN) -
Cytochrome b-c1 complex subunit 1, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
480 a.a.
446 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P23004
(QCR2_BOVIN) -
Cytochrome b-c1 complex subunit 2, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
453 a.a.
423 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00157
(CYB_BOVIN) -
Cytochrome b from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
379 a.a.
378 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00125
(CY1_BOVIN) -
Cytochrome c1, heme protein, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
325 a.a.
241 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P13272
(UCRI_BOVIN) -
Cytochrome b-c1 complex subunit Rieske, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
274 a.a.
196 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00129
(QCR7_BOVIN) -
Cytochrome b-c1 complex subunit 7 from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
111 a.a.
106 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P13271
(QCR8_BOVIN) -
Cytochrome b-c1 complex subunit 8 from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
82 a.a.
75 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P00126
(QCR6_BOVIN) -
Cytochrome b-c1 complex subunit 6, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
91 a.a.
64 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|

|

|
|
P13272
(UCRI_BOVIN) -
Cytochrome b-c1 complex subunit Rieske, mitochondrial from Bos taurus
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
274 a.a.
57 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
 |
|
|
 |
 |
 |
 |
Enzyme class 2:
|
 |
Chains A, B, G, H, J, K:
E.C.1.10.2.2
- Transferred entry: 7.1.1.8.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
Quinol + 2 ferricytochrome c = quinone + 2 ferrocytochrome c + 2 H+
|
 |
 |
 |
 |
 |
Quinol
|
+
|
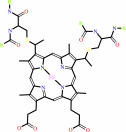 2
×
ferricytochrome c
2
×
ferricytochrome c
|
=
|
 quinone
quinone
|
+
|
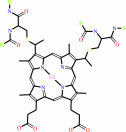 2
×
ferrocytochrome c
2
×
ferrocytochrome c
|
+
|
2
×
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Enzyme class 3:
|
 |
Chains D, E, I:
E.C.7.1.1.8
- quinol--cytochrome-c reductase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a quinol + 2 Fe(III)-[cytochrome c](out) = a quinone + 2 Fe(II)- [cytochrome c](out) + 2 H(+)(out)
|
 |
 |
 |
 |
 |
quinol
|
+
|
2
×
Fe(III)-[cytochrome c](out)
|
=
|
 quinone
quinone
|
+
|
2
×
Fe(II)- [cytochrome c](out)
|
+
|
2
×
H(+)(out)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Note, where more than one E.C. class is given (as above), each may
correspond to a different protein domain or, in the case of polyprotein
precursors, to a different mature protein.
|
|
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
103:13045-13050
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Surface-modulated motion switch: capture and release of iron-sulfur protein in the cytochrome bc1 complex.
|
|
L.Esser,
X.Gong,
S.Yang,
L.Yu,
C.A.Yu,
D.Xia.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
In the cytochrome bc(1) complex, the swivel motion of the iron-sulfur protein
(ISP) between two redox sites constitutes a key component of the mechanism that
achieves the separation of the two electrons in a substrate molecule at the
quinol oxidation (Q(o)) site. The question remaining is how the motion of ISP is
controlled so that only one electron enters the thermodynamically favorable
chain via ISP. An analysis of eight structures of mitochondrial bc(1) with bound
Q(o) site inhibitors revealed that the presence of inhibitors causes a
bidirectional repositioning of the cd1 helix in the cytochrome b subunit. As the
cd1 helix forms a major part of the ISP binding crater, any positional shift of
this helix modulates the ability of cytochrome b to bind ISP. The analysis also
suggests a mechanism for reversal of the ISP fixation when the shape
complementarity is significantly reduced after a positional reorientation of the
reaction product quinone. The importance of shape complementarity in this
mechanism was confirmed by functional studies of bc(1) mutants and by a
structure determination of the bacterial form of bc(1). A mechanism for the high
fidelity of the bifurcated electron transfer is proposed.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Prosthetic groups and subunit structures of the cyt
bc[1] complex. (A) Arrangement of prosthetic groups in the
dimeric bc[1] complex and illustration of the electron
bifurcation at the Q[o] site. The b[L], b[H], and c[1] heme
groups are shown as ball-and-stick models, and the [2Fe2S]
clusters are depicted as cpk models. Carbon atoms, black;
nitrogen, blue; oxygen, red; sulfur, yellow; iron, brown. The
Q[o] pockets near the IMS side of the membrane and the Q[i]
pockets near the matrix side are labeled and shaded in gray. Cyt
c is shown as a gray shaded oval. Distances between redox
centers are given on the left half of the diagram, and the redox
potential for each center is given on the right. The high- and
low-potential ET paths are depicted with red and green arrows,
respectively. Circles in pink and light green within the Q[o]
pockets are hypothesized distal-QH[2] and proximal-Q binding
sites, respectively. (B) Ribbon diagram of the dimeric cyt b,
cyt c[1], and ISP subunit in the mitochondrial bc[1] complex.
Two symmetry-related cyt b subunits are shown (green and light
green). The eight TM helices of cyt b are denoted with letters
A–H. Helices A–E form one bundle in which the two b-type
hemes (b[L] and b[H] in ball-and-stick models) reside; helices
F–H form the other bundle. The ISP subunit (yellow and red for
the symmetry pair) has an extrinsic soluble domain with a
[2Fe2S] cluster at its tip, connecting to a TM segment by a
flexible neck. The extrinsic domain of cyt c[1] (blue and
magenta for the symmetry pair) with its heme group is rigidly
attached to its TM helix. The locations for the two active sites
(Q[o] and Q[i]) per monomer in cyt b are labeled. The surface
depression in cyt b at the IMS side of the membrane is labeled
as the ISP-docking crater.
|
 |
Figure 3.
Fig. 3. Control of the ISP-ED motion switch and the
proposed mechanism for electron bifurcation at the Q[o] pocket.
The structural components necessary for the control of ISP
conformational switch are illustrated in this cartoon rendition
of the Q[o] pocket. The PEWY motif and cd1 helix in gray
represent a native (Rest) configuration. The ISP in yellow and
magenta are of oxidized and reduced form, respectively. The PEWY
in blue stands for the open configuration with a bound Q[o] site
inhibitor. The cd1 helix in red symbolizes the conformation
(On/+) in the presence of a Pf inhibitor occupying the distal
site (pink), and the cd1 helix in green shows the conformation
(Off/–) when a Pm inhibitor is taking the proximal site
(purple). Cyt c[1] and heme b[L] are also shown.
|
 |
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
E.Cieluch,
K.Pietryga,
M.Sarewicz,
and
A.Osyczka
(2010).
Visualizing changes in electron distribution in coupled chains of cytochrome bc(1) by modifying barrier for electron transfer between the FeS cluster and heme c(1).
|
| |
Biochim Biophys Acta,
1797,
296-303.
|
 |
|
|
|
|
 |
K.McLuskey,
A.W.Roszak,
Y.Zhu,
and
N.W.Isaacs
(2010).
Crystal structures of all-alpha type membrane proteins.
|
| |
Eur Biophys J,
39,
723-755.
|
 |
|
|
|
|
 |
K.R.Vinothkumar,
and
R.Henderson
(2010).
Structures of membrane proteins.
|
| |
Q Rev Biophys,
43,
65.
|
 |
|
|
|
|
 |
J.W.Cooley,
D.W.Lee,
and
F.Daldal
(2009).
Across membrane communication between the Q(o) and Q(i) active sites of cytochrome bc(1).
|
| |
Biochemistry,
48,
1888-1899.
|
 |
|
|
|
|
 |
M.Sarewicz,
M.Dutka,
W.Froncisz,
and
A.Osyczka
(2009).
Magnetic interactions sense changes in distance between heme b(L) and the iron-sulfur cluster in cytochrome bc(1).
|
| |
Biochemistry,
48,
5708-5720.
|
 |
|
|
|
|
 |
Q.L.Chen,
X.S.Tang,
W.J.Yao,
and
S.Q.Lu
(2009).
Bioinformatics analysis of the complete sequences of cytochrome b of Takydromus sylvaticus and modeling the tertiary structure of encoded protein.
|
| |
Int J Biol Sci,
5,
596-602.
|
 |
|
|
|
|
 |
R.Covian,
and
B.L.Trumpower
(2009).
The rate-limiting step in the cytochrome bc1 complex (Ubiquinol-Cytochrome c Oxidoreductase) is not changed by inhibition of cytochrome b-dependent deprotonation: implications for the mechanism of ubiquinol oxidation at center P of the bc1 complex.
|
| |
J Biol Chem,
284,
14359-14367.
|
 |
|
|
|
|
 |
A.R.Crofts,
J.T.Holland,
D.Victoria,
D.R.Kolling,
S.A.Dikanov,
R.Gilbreth,
S.Lhee,
R.Kuras,
and
M.G.Kuras
(2008).
The Q-cycle reviewed: How well does a monomeric mechanism of the bc(1) complex account for the function of a dimeric complex?
|
| |
Biochim Biophys Acta,
1777,
1001-1019.
|
 |
|
|
|
|
 |
C.Cai,
L.Chang,
W.Li,
and
W.Liu
(2008).
Effects of hyperoxia on mitochondrial multienzyme complex III and V in premature newborn rat lung.
|
| |
J Huazhong Univ Sci Technolog Med Sci,
28,
207-210.
|
 |
|
|
|
|
 |
D.E.Chandler,
J.Hsin,
C.B.Harrison,
J.Gumbart,
and
K.Schulten
(2008).
Intrinsic curvature properties of photosynthetic proteins in chromatophores.
|
| |
Biophys J,
95,
2822-2836.
|
 |
|
|
|
|
 |
D.Xia,
L.Esser,
M.Elberry,
F.Zhou,
L.Yu,
and
C.A.Yu
(2008).
The road to the crystal structure of the cytochrome bc (1) complex from the anoxigenic, photosynthetic bacterium Rhodobacter sphaeroides.
|
| |
J Bioenerg Biomembr,
40,
485-492.
|
 |
|
|
|
|
 |
H.W.Ma,
S.Yang,
L.Yu,
and
C.A.Yu
(2008).
Formation of engineered intersubunit disulfide bond in cytochrome bc1 complex disrupts electron transfer activity in the complex.
|
| |
Biochim Biophys Acta,
1777,
317-326.
|
 |
|
|
|
|
 |
P.J.Crowley,
E.A.Berry,
T.Cromartie,
F.Daldal,
C.R.Godfrey,
D.W.Lee,
J.E.Phillips,
A.Taylor,
and
R.Viner
(2008).
The role of molecular modeling in the design of analogues of the fungicidal natural products crocacins A and D.
|
| |
Bioorg Med Chem,
16,
10345-10355.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
R.R.da Fonseca,
W.E.Johnson,
S.J.O'Brien,
M.J.Ramos,
and
A.Antunes
(2008).
The adaptive evolution of the mammalian mitochondrial genome.
|
| |
BMC Genomics,
9,
119.
|
 |
|
|
|
|
 |
A.Y.Mulkidjanian
(2007).
Proton translocation by the cytochrome bc1 complexes of phototrophic bacteria: introducing the activated Q-cycle.
|
| |
Photochem Photobiol Sci,
6,
19-34.
|
 |
|
|
|
|
 |
D.Xia,
L.Esser,
L.Yu,
and
C.A.Yu
(2007).
Structural basis for the mechanism of electron bifurcation at the quinol oxidation site of the cytochrome bc1 complex.
|
| |
Photosynth Res,
92,
17-34.
|
 |
|
|
|
|
 |
E.Yamashita,
H.Zhang,
and
W.A.Cramer
(2007).
Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn.
|
| |
J Mol Biol,
370,
39-52.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
K.Ogawa,
T.Sonoyama,
T.Takeda,
S.Ichiki,
S.Nakamura,
Y.Kobayashi,
S.Uchiyama,
K.Nakasone,
S.J.Takayama,
H.Mita,
Y.Yamamoto,
and
Y.Sambongi
(2007).
Roles of a short connecting disulfide bond in the stability and function of psychrophilic Shewanella violacea cytochrome c (5)*.
|
| |
Extremophiles,
11,
797-807.
|
 |
|
|
|
|
 |
L.Giachini,
F.Francia,
G.Veronesi,
D.W.Lee,
F.Daldal,
L.S.Huang,
E.A.Berry,
T.Cocco,
S.Papa,
F.Boscherini,
and
G.Venturoli
(2007).
X-Ray absorption studies of Zn2+ binding sites in bacterial, avian, and bovine cytochrome bc1 complexes.
|
| |
Biophys J,
93,
2934-2951.
|
 |
|
|
|
|
 |
S.Devanathan,
Z.Salamon,
G.Tollin,
J.C.Fitch,
T.E.Meyer,
E.A.Berry,
and
M.A.Cusanovich
(2007).
Plasmon waveguide resonance spectroscopic evidence for differential binding of oxidized and reduced Rhodobacter capsulatus cytochrome c2 to the cytochrome bc1 complex mediated by the conformation of the Rieske iron-sulfur protein.
|
| |
Biochemistry,
46,
7138-7145.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|

| |

