 |
PDBsum entry 2fpy

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2fpy
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.3.5.2
- dihydroorotate dehydrogenase (quinone).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
(S)-dihydroorotate + a quinone = orotate + a quinol
|
 |
 |
 |
 |
 |
(S)-dihydroorotate
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
 quinone
quinone
|
=
|
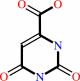 orotate
orotate
|
+
|
quinol
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
FMN
|
 |
 |
 |
 |
 |
FMN
Bound ligand (Het Group name =
FMN)
corresponds exactly
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
J Med Chem
49:1239-1247
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Dual binding mode of a novel series of DHODH inhibitors.
|
|
R.Baumgartner,
M.Walloschek,
M.Kralik,
A.Gotschlich,
S.Tasler,
J.Mies,
J.Leban.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Human dihydroorotate dehydrogenase (DHODH) represents an important target for
the treatment of hyperproliferative and inflammatory diseases. In the cell DHODH
catalyzes the rate-limiting step of the de novo pyrimidine biosynthesis. DHODH
inhibition results in beneficial immunosuppressant and antiproliferative effects
in diseases such as rheumatoid arthritis. Here, we present high-resolution X-ray
structures of human DHODH in complex with a novel class of low molecular weight
compounds that inhibit the enzyme in the nanomolar range. Some compounds showed
an interesting dual binding mode within the same cocrystal strongly depending on
the nature of chemical substitution. Measured in vitro activity data correlated
with the prevailing mode of binding and explained the observed
structure-activity relationship. Additionally, the X-ray data confirmed the
competitive nature of the inhibitors toward the putative ubiquinone binding site
and will guide structure-based design and synthesis of molecules with higher
activity.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Bonavia,
M.Franti,
E.Pusateri Keaney,
K.Kuhen,
M.Seepersaud,
B.Radetich,
J.Shao,
A.Honda,
J.Dewhurst,
K.Balabanis,
J.Monroe,
K.Wolff,
C.Osborne,
L.Lanieri,
K.Hoffmaster,
J.Amin,
J.Markovits,
M.Broome,
E.Skuba,
I.Cornella-Taracido,
G.Joberty,
T.Bouwmeester,
L.Hamann,
J.A.Tallarico,
R.Tommasi,
T.Compton,
and
S.M.Bushell
(2011).
Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV).
|
| |
Proc Natl Acad Sci U S A,
108,
6739-6744.
|
 |
|
|
|
|
 |
I.Fritzson,
B.Svensson,
S.Al-Karadaghi,
B.Walse,
U.Wellmar,
U.J.Nilsson,
D.da Graça Thrige,
and
S.Jönsson
(2010).
Inhibition of human DHODH by 4-hydroxycoumarins, fenamic acids, and N-(alkylcarbonyl)anthranilic acids identified by structure-guided fragment selection.
|
| |
ChemMedChem,
5,
608-617.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
M.A.Phillips,
and
P.K.Rathod
(2010).
Plasmodium dihydroorotate dehydrogenase: a promising target for novel anti-malarial chemotherapy.
|
| |
Infect Disord Drug Targets,
10,
226-239.
|
 |
|
|
|
|
 |
C.C.Lee,
R.J.Fitzmaurice,
and
S.Caddick
(2009).
3,5-Isoxazoles from alpha-bromo-pentafluorophenyl vinylsulfonates: synthesis of sulfonates and sulfonamides.
|
| |
Org Biomol Chem,
7,
4349-4351.
|
 |
|
|
|
|
 |
R.L.Kow,
J.R.Whicher,
C.A.McDonald,
B.A.Palfey,
and
R.L.Fagan
(2009).
Disruption of the proton relay network in the class 2 dihydroorotate dehydrogenase from Escherichia coli.
|
| |
Biochemistry,
48,
9801-9809.
|
 |
|
|
|
|
 |
X.Deng,
R.Gujjar,
F.El Mazouni,
W.Kaminsky,
N.A.Malmquist,
E.J.Goldsmith,
P.K.Rathod,
and
M.A.Phillips
(2009).
Structural plasticity of malaria dihydroorotate dehydrogenase allows selective binding of diverse chemical scaffolds.
|
| |
J Biol Chem,
284,
26999-27009.
|
 |
|
PDB codes:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

