 |
PDBsum entry 2fpc

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.5.99.13
- strictosidine synthase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
3alpha(S)-strictosidine + H2O = secologanin + tryptamine
|
 |
 |
 |
 |
 |
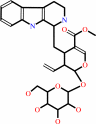 3alpha(S)-strictosidine
3alpha(S)-strictosidine
|
+
|
H2O
|
=
|
secologanin
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
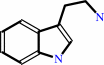 tryptamine
tryptamine
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Plant Cell
18:907-920
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
The structure of Rauvolfia serpentina strictosidine synthase is a novel six-bladed beta-propeller fold in plant proteins.
|
|
X.Ma,
S.Panjikar,
J.Koepke,
E.Loris,
J.Stöckigt.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The enzyme strictosidine synthase (STR1) from the Indian medicinal plant
Rauvolfia serpentina is of primary importance for the biosynthetic pathway of
the indole alkaloid ajmaline. Moreover, STR1 initiates all biosynthetic pathways
leading to the entire monoterpenoid indole alkaloid family representing an
enormous structural variety of approximately 2000 compounds in higher plants.
The crystal structures of STR1 in complex with its natural substrates tryptamine
and secologanin provide structural understanding of the observed substrate
preference and identify residues lining the active site surface that contact the
substrates. STR1 catalyzes a Pictet-Spengler-type reaction and represents a
novel six-bladed beta-propeller fold in plant proteins. Structure-based sequence
alignment revealed a common repetitive sequence motif (three hydrophobic
residues are followed by a small residue and a hydrophilic residue), indicating
a possible evolutionary relationship between STR1 and several sequence-unrelated
six-bladed beta-propeller structures. Structural analysis and site-directed
mutagenesis experiments demonstrate the essential role of Glu-309 in catalysis.
The data will aid in deciphering the details of the reaction mechanism of STR1
as well as other members of this enzyme family.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
L.Yang,
and
J.Stöckigt
(2010).
Trends for diverse production strategies of plant medicinal alkaloids.
|
| |
Nat Prod Rep,
27,
1469-1479.
|
 |
|
|
|
|
 |
H.Y.Lee,
N.Yerkes,
and
S.E.O'Connor
(2009).
Aza-tryptamine substrates in monoterpene indole alkaloid biosynthesis.
|
| |
Chem Biol,
16,
1225-1229.
|
 |
|
|
|
|
 |
K.S.Ryan,
and
B.S.Moore
(2009).
Alkaloid biosynthesis takes root.
|
| |
Nat Chem Biol,
5,
140-141.
|
 |
|
|
|
|
 |
K.Yonekura-Sakakibara,
and
K.Saito
(2009).
Functional genomics for plant natural product biosynthesis.
|
| |
Nat Prod Rep,
26,
1466-1487.
|
 |
|
|
|
|
 |
P.Bernhardt,
N.Yerkes,
and
S.E.O'Connor
(2009).
Bypassing stereoselectivity in the early steps of alkaloid biosynthesis.
|
| |
Org Biomol Chem,
7,
4166-4168.
|
 |
|
|
|
|
 |
W.Runguphan,
and
S.E.O'Connor
(2009).
Metabolic reprogramming of periwinkle plant culture.
|
| |
Nat Chem Biol,
5,
151-153.
|
 |
|
|
|
|
 |
Y.Lu,
H.Wang,
W.Wang,
Z.Qian,
L.Li,
J.Wang,
G.Zhou,
and
G.Kai
(2009).
Molecular characterization and expression analysis of a new cDNA encoding strictosidine synthase from Ophiorrhiza japonica.
|
| |
Mol Biol Rep,
36,
1845-1852.
|
 |
|
|
|
|
 |
J.J.Maresh,
L.A.Giddings,
A.Friedrich,
E.A.Loris,
S.Panjikar,
B.L.Trout,
J.Stöckigt,
B.Peters,
and
S.E.O'Connor
(2008).
Strictosidine synthase: mechanism of a Pictet-Spengler catalyzing enzyme.
|
| |
J Am Chem Soc,
130,
710-723.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Ziegler,
and
P.J.Facchini
(2008).
Alkaloid biosynthesis: metabolism and trafficking.
|
| |
Annu Rev Plant Biol,
59,
735-769.
|
 |
|
|
|
|
 |
P.J.Facchini,
and
V.De Luca
(2008).
Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants.
|
| |
Plant J,
54,
763-784.
|
 |
|
|
|
|
 |
B.O.Bachmann
(2007).
Foundations for directed alkaloid biosynthesis.
|
| |
Chem Biol,
14,
875-876.
|
 |
|
|
|
|
 |
E.A.Loris,
S.Panjikar,
M.Ruppert,
L.Barleben,
M.Unger,
H.Schübel,
and
J.Stöckigt
(2007).
Structure-based engineering of strictosidine synthase: auxiliary for alkaloid libraries.
|
| |
Chem Biol,
14,
979-985.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
J.Stöckigt,
and
S.Panjikar
(2007).
Structural biology in plant natural product biosynthesis--architecture of enzymes from monoterpenoid indole and tropane alkaloid biosynthesis.
|
| |
Nat Prod Rep,
24,
1382-1400.
|
 |
|
|
|
|
 |
M.C.Galan,
E.McCoy,
and
S.E.O'Connor
(2007).
Chemoselective derivatization of alkaloids in periwinkle.
|
| |
Chem Commun (Camb),
(),
3249-3251.
|
 |
|
|
|
|
 |
P.Bernhardt,
E.McCoy,
and
S.E.O'Connor
(2007).
Rapid identification of enzyme variants for reengineered alkaloid biosynthesis in periwinkle.
|
| |
Chem Biol,
14,
888-897.
|
 |
|
|
|
|
 |
C.Rosenthal,
U.Mueller,
S.Panjikar,
L.Sun,
M.Ruppert,
Y.Zhao,
and
J.Stöckigt
(2006).
Expression, purification, crystallization and preliminary X-ray analysis of perakine reductase, a new member of the aldo-keto reductase enzyme superfamily from higher plants.
|
| |
Acta Crystallogr Sect F Struct Biol Cryst Commun,
62,
1286-1289.
|
 |
|
|
|
|
 |
S.E.O'Connor,
and
J.J.Maresh
(2006).
Chemistry and biology of monoterpene indole alkaloid biosynthesis.
|
| |
Nat Prod Rep,
23,
532-547.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
code is
shown on the right.
|
 |

