 |
PDBsum entry 2fix

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.3.1.3.11
- fructose-bisphosphatase.
|
|
 |
 |
 |
 |
 |

Pathway:
|
 |
Pentose Phosphate Pathway (later stages)
|
 |
 |
 |
 |
 |
Reaction:
|
 |
beta-D-fructose 1,6-bisphosphate + H2O = beta-D-fructose 6-phosphate + phosphate
|
 |
 |
 |
 |
 |
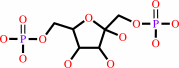 beta-D-fructose 1,6-bisphosphate
beta-D-fructose 1,6-bisphosphate
|
+
|
H2O
|
=
|
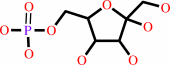 beta-D-fructose 6-phosphate
beta-D-fructose 6-phosphate
|
+
|
 phosphate
phosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Bioorg Med Chem Lett
16:1807-1810
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Benzoxazole benzenesulfonamides as allosteric inhibitors of fructose-1,6-bisphosphatase.
|
|
C.Lai,
R.J.Gum,
M.Daly,
E.H.Fry,
C.Hutchins,
C.Abad-Zapatero,
T.W.von Geldern.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
A series of novel benzoxazole benzenesulfonamides was synthesized as inhibitors
of fructose-1,6-bisphosphatase (FBPase-1). Extensive SAR studies led to a potent
inhibitor, 53, with an IC(50) of 0.57microM. Compound 17 exhibited excellent
bioavailability and a good pharmacokinetic profile in rats.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Rolfe,
and
P.R.Hanson
(2009).
Microwave-assisted sequential one-pot protocol to benzothiadiazin-3-one-1,1-dioxides via a copper-catalyzed N-arylation strategy.
|
| |
Tetrahedron Lett,
50,
6935-6937.
|
 |
|
|
|
|
 |
M.L.Mohler,
Y.He,
Z.Wu,
D.J.Hwang,
and
D.D.Miller
(2009).
Recent and emerging anti-diabetes targets.
|
| |
Med Res Rev,
29,
125-195.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

