 |
PDBsum entry 2e89

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Ligase
|
 |
|
Title:
|
 |
Crystal structure of aquifex aeolicus tils in a complex with atp, magnesium ion, and l-lysine
|
|
Structure:
|
 |
tRNA(ile)-lysidine synthase. Chain: a, b, c, d. Synonym: tRNA(ile)-lysidine synthetase, tRNA(ile)-2-lysyl-cytidine synthase. Engineered: yes
|
|
Source:
|
 |
Aquifex aeolicus. Organism_taxid: 63363. Expressed in: escherichia coli bl21(de3). Expression_system_taxid: 469008.
|
|
Resolution:
|
 |
|
2.50Å
|
R-factor:
|
0.229
|
R-free:
|
0.274
|
|
|
Authors:
|
 |
M.Kuratani,Y.Yoshikawa,S.Takahashi,S.Yokoyama,Riken Structural Genomics/proteomics Initiative (Rsgi)
|
Key ref:

|
 |
M.Kuratani
et al.
(2007).
Structural basis of the initial binding of tRNA(Ile) lysidine synthetase TilS with ATP and L-lysine.
Structure,
15,
1642-1653.
PubMed id:
DOI:

|
 |
|
Date:
|
 |
|
19-Jan-07
|
Release date:
|
13-Nov-07
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
O67728
(TILS_AQUAE) -
tRNA(Ile)-lysidine synthase from Aquifex aeolicus (strain VF5)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
317 a.a.
317 a.a.
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.3.4.19
- tRNA(Ile)-lysidine synthetase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
cytidine34 in tRNA(Ile2) + L-lysine + ATP = lysidine34 in tRNA(Ile2) + AMP + diphosphate + H+
|
 |
 |
 |
 |
 |
cytidine(34) in tRNA(Ile2)
Bound ligand (Het Group name = )
corresponds exactly
|
+
|
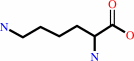 L-lysine
L-lysine
|
+
|
ATP
Bound ligand (Het Group name = )
corresponds exactly
|
=
|
lysidine(34) in tRNA(Ile2)
|
+
|
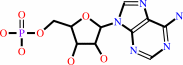 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
+
|
H(+)
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
15:1642-1653
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural basis of the initial binding of tRNA(Ile) lysidine synthetase TilS with ATP and L-lysine.
|
|
M.Kuratani,
Y.Yoshikawa,
Y.Bessho,
K.Higashijima,
T.Ishii,
R.Shibata,
S.Takahashi,
K.Yutani,
S.Yokoyama.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
In the bacterial genetic-code system, the codon AUA is decoded as isoleucine by
tRNA(Ile)(2) with the lysidine residue at the wobble position. Lysidine is
derived from cytidine, with ATP and L-lysine, by tRNA(Ile) lysidine synthetase
(TilS), which is an N-type ATP pyrophosphatase. In this study, we determined the
crystal structure of Aquifex aeolicus TilS, complexed with ATP, Mg2+, and
L-lysine, at 2.5 A resolution. The presence of the TilS-specific subdomain
causes the active site to have two separate gateways, a large hole and a narrow
tunnel on the opposite side. ATP is bound inside the hole, and L-lysine is bound
at the entrance of the tunnel. The conserved Asp36 in the PP-motif coordinates
Mg2+. In these initial binding modes, the ATP, Mg2+, and L-lysine are held far
apart from each other, but they seem to be brought together for the reaction
upon cytidine binding, with putative structural changes of the complex.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 2.
Figure 2. Overall Structure
(A–C) |Fo| − |Fc|
simulated annealing omit maps. (A) The electron density of the
AMPPNP (blue, contoured at 4σ). (B) The electron densities of
the ATP (blue, 5.5σ) and the Mg^2+ (brown, 4σ). The continuity
of the electron densities of the Mg^2+, Asp36, and Asp137 is
shown by omitting these three molecules (yellow, 3σ). (C) The
electron densities of the L-lysine (blue, 3.5σ).
(D)
Ribbon model of the TilS dimer. Two subunits (molecules A and B
of TilS/ATP/Mg/Lys) are colored pink and cyan, respectively. The
ATP and L-lysine molecules are shown by stick models.
(E) A
stereoview of the TilS monomer (molecule B of TilS/ATP/Mg/Lys).
The N-terminal domain (NTD), the TilS-specific subdomain (TSD),
the linker, and the C-terminal domain (CTD1) are colored pink,
yellow, green, and cyan, respectively. The graphic figures in
this paper were prepared with CueMol
(http://cuemol.sourceforge.jp/en/) and were rendered with POVRAY
(http://www.povray.org/).
|
 |
Figure 7.
Figure 7. ATP Recognition
(A) The amino acid residues
that recognize the ATP and Mg^2+ (stereoview). The ATP is shown
by a stick model. The Mg^2+ and water molecules are shown as
gray and red spheres, respectively. Hydrogen bonds are shown as
dotted lines.
(B) Recognition of the AMPPNP, shown as in
(A). The nitrogen atom between the P[β] and P[γ] atoms is
colored blue.
(C) AMP and pyrophosphate binding by E. coli
GMP synthetase, depicted as in (A).
(D and E) Extended (D)
and U-shaped (E) ATP conformations in the structures of the E.
coli argininosuccinate synthetase complexed with ATP (D) and
with both ATP and citrulline (E), respectively. The side chain
of Asp22 in (D) is missing in the coordinates (1KP2).
(F)
Comparison of the ATP conformation. The U-shaped ATP, with three
manganese ions (Mn1, Mn2, and Mn3) in the structure of LysU (PDB
code: 1E24) was superposed based on the adenine ring. The
phosphate atoms are colored orange, and the manganese ions are
colored magenta.
(G) Docking model of TilS and the cytidine
residue of tRNA^Ile[2] (stereoview). The phosphate atoms of the
ATP and the side chain of Asp36 were moved manually. The
L-lysine was moved manually, and the model structure is colored
light gray and is indicated as (L-lysine).
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Structure
(2007,
15,
1642-1653)
copyright 2007.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Guelorget,
and
B.Golinelli-Pimpaneau
(2011).
Mechanism-based strategies for trapping and crystallizing complexes of RNA-modifying enzymes.
|
| |
Structure,
19,
282-291.
|
 |
|
|
|
|
 |
C.Fabret,
E.Dervyn,
B.Dalmais,
A.Guillot,
C.Marck,
H.Grosjean,
and
P.Noirot
(2011).
Life without the essential bacterial tRNA(Ile2) -lysidine synthetase TilS: a case of tRNA gene recruitment in Bacillus subtilis.
|
| |
Mol Microbiol,
80,
1062-1074.
|
 |
|
|
|
|
 |
T.Osawa,
S.Kimura,
N.Terasaka,
H.Inanaga,
T.Suzuki,
and
T.Numata
(2011).
Structural basis of tRNA agmatinylation essential for AUA codon decoding.
|
| |
Nat Struct Mol Biol,
18,
1275-1280.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
S.P.Salowe,
J.Wiltsie,
J.C.Hawkins,
and
L.M.Sonatore
(2009).
The catalytic flexibility of tRNAIle-lysidine synthetase can generate alternative tRNA substrates for isoleucyl-tRNA synthetase.
|
| |
J Biol Chem,
284,
9656-9662.
|
 |
|
|
|
|
 |
R.Ishitani,
S.Yokoyama,
and
O.Nureki
(2008).
Structure, dynamics, and function of RNA modification enzymes.
|
| |
Curr Opin Struct Biol,
18,
330-339.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

