 |
PDBsum entry 2e7z

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.4.2.1.112
- acetylene hydratase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
acetaldehyde = acetylene + H2O
|
 |
 |
 |
 |
 |
acetaldehyde
Bound ligand (Het Group name = )
matches with 75.00% similarity
|
=
|
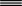 acetylene
acetylene
|
+
|
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
Cofactor:
|
 |
Iron-sulfur; Mo-bis(molybdopterin guanine dinucleotide); W cation
|
 |
 |
 |
 |
 |
 Iron-sulfur
Iron-sulfur
|
Mo-bis(molybdopterin guanine dinucleotide)
|
W cation
|
|
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Proc Natl Acad Sci U S A
104:3073-3077
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structure of the non-redox-active tungsten/[4Fe:4S] enzyme acetylene hydratase.
|
|
G.B.Seiffert,
G.M.Ullmann,
A.Messerschmidt,
B.Schink,
P.M.Kroneck,
O.Einsle.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
The tungsten-iron-sulfur enzyme acetylene hydratase stands out from its class
because it catalyzes a nonredox reaction, the hydration of acetylene to
acetaldehyde. Sequence comparisons group the protein into the dimethyl sulfoxide
reductase family, and it contains a bis-molybdopterin guanine
dinucleotide-ligated tungsten atom and a cubane-type [4Fe:4S] cluster. The
crystal structure of acetylene hydratase at 1.26 A now shows that the tungsten
center binds a water molecule that is activated by an adjacent aspartate
residue, enabling it to attack acetylene bound in a distinct, hydrophobic
pocket. This mechanism requires a strong shift of pK(a) of the aspartate, caused
by a nearby low-potential [4Fe:4S] cluster. To access this previously
unrecognized W-Asp active site, the protein evolved a new substrate channel
distant from where it is found in other molybdenum and tungsten enzymes.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 1.
Fig. 1. Overall structure of acetylene hydratase from P.
acetylenicus. The stereo representation shows an orientation
viewing down the active site channel as seen in Fig. 4A.
|
 |
Figure 3.
Fig. 3. Cofactors and active site of AH. (A) The tungsten
atom (blue) is coordinated by the dithiolene groups of both MGD
cofactors and the side chain of Cys-141. A water molecule
completes the slightly distorted octahedral geometry. This water
is also hydrogen-bonded to Asp-13, a residue adjacent to the
[4Fe:4S] cluster ligand Cys-12. (B) Above the bound water
molecule, a ring of hydrophobic residues forms the bottom of the
active site access channel (see Fig. 4).
|
 |
|
|
| |
Figures were
selected
by the author.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
J.Jin,
A.J.Straathof,
M.W.Pinkse,
and
U.Hanefeld
(2011).
Purification, characterization, and cloning of a bifunctional molybdoenzyme with hydratase and alcohol dehydrogenase activity.
|
| |
Appl Microbiol Biotechnol,
89,
1831-1840.
|
 |
|
|
|
|
 |
J.Jin,
and
U.Hanefeld
(2011).
The selective addition of water to C=C bonds; enzymes are the best chemists.
|
| |
Chem Commun (Camb),
47,
2502-2510.
|
 |
|
|
|
|
 |
P.V.Bernhardt
(2011).
Exploiting the versatility and selectivity of Mo enzymes with electrochemistry.
|
| |
Chem Commun (Camb),
47,
1663-1673.
|
 |
|
|
|
|
 |
A.Döring,
and
C.Schulzke
(2010).
Tungsten's redox potential is more temperature sensitive than that of molybdenum.
|
| |
Dalton Trans,
39,
5623-5629.
|
 |
|
|
|
|
 |
M.A.Vincent,
I.H.Hillier,
G.Periyasamy,
and
N.A.Burton
(2010).
A DFT study of the possible role of vinylidene and carbene intermediates in the mechanism of the enzyme acetylene hydratase.
|
| |
Dalton Trans,
39,
3816-3822.
|
 |
|
|
|
|
 |
M.S.Till,
and
G.M.Ullmann
(2010).
McVol - a program for calculating protein volumes and identifying cavities by a Monte Carlo algorithm.
|
| |
J Mol Model,
16,
419-429.
|
 |
|
|
|
|
 |
R.Z.Liao,
J.G.Yu,
and
F.Himo
(2010).
Mechanism of tungsten-dependent acetylene hydratase from quantum chemical calculations.
|
| |
Proc Natl Acad Sci U S A,
107,
22523-22527.
|
 |
|
|
|
|
 |
M.J.Romão
(2009).
Molybdenum and tungsten enzymes: a crystallographic and mechanistic overview.
|
| |
Dalton Trans,
(),
4053-4068.
|
 |
|
|
|
|
 |
S.Groysman,
and
R.H.Holm
(2009).
Biomimetic chemistry of iron, nickel, molybdenum, and tungsten in sulfur-ligated protein sites.
|
| |
Biochemistry,
48,
2310-2320.
|
 |
|
|
|
|
 |
A.Bashan,
and
A.Yonath
(2008).
The linkage between ribosomal crystallography, metal ions, heteropolytungstates and functional flexibility.
|
| |
J Mol Struct,
890,
289-294.
|
 |
|
|
|
|
 |
H.Sugimoto,
and
H.Tsukube
(2008).
Chemical analogues relevant to molybdenum and tungsten enzyme reaction centres toward structural dynamics and reaction diversity.
|
| |
Chem Soc Rev,
37,
2609-2619.
|
 |
|
|
|
|
 |
J.R.Andreesen,
and
K.Makdessi
(2008).
Tungsten, the surprisingly positively acting heavy metal element for prokaryotes.
|
| |
Ann N Y Acad Sci,
1125,
215-229.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
|
 |

