 |
PDBsum entry 2cv2

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.6.1.1.17
- glutamate--tRNA ligase.
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
tRNA(Glu) + L-glutamate + ATP = L-glutamyl-tRNA(Glu) + AMP + diphosphate
|
 |
 |
 |
 |
 |
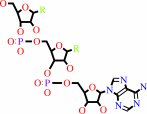 tRNA(Glu)
tRNA(Glu)
|
+
|
 L-glutamate
L-glutamate
|
+
|
 ATP
ATP
|
=
|
L-glutamyl-tRNA(Glu)
Bound ligand (Het Group name = )
matches with 52.78% similarity
|
+
|
 AMP
AMP
|
+
|
 diphosphate
diphosphate
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
DOI no:
|
Structure
14:1791-1799
(2006)
|
|
PubMed id:
|
|
|
|

|
| |
|
Structural bases of transfer RNA-dependent amino acid recognition and activation by glutamyl-tRNA synthetase.
|
|
S.Sekine,
M.Shichiri,
S.Bernier,
R.Chênevert,
J.Lapointe,
S.Yokoyama.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Glutamyl-tRNA synthetase (GluRS) is one of the aminoacyl-tRNA synthetases that
require the cognate tRNA for specific amino acid recognition and activation. We
analyzed the role of tRNA in amino acid recognition by crystallography. In the
GluRS*tRNA(Glu)*Glu structure, GluRS and tRNA(Glu) collaborate to form a highly
complementary L-glutamate-binding site. This collaborative site is functional,
as it is formed in the same manner in pretransition-state mimic,
GluRS*tRNA(Glu)*ATP*Eol (a glutamate analog), and posttransition-state mimic,
GluRS*tRNA(Glu)*ESA (a glutamyl-adenylate analog) structures. In contrast, in
the GluRS*Glu structure, only GluRS forms the amino acid-binding site, which is
defective and accounts for the binding of incorrect amino acids, such as
D-glutamate and L-glutamine. Therefore, tRNA(Glu) is essential for formation of
the completely functional binding site for L-glutamate. These structures,
together with our previously described structures, reveal that tRNA plays a
crucial role in accurate positioning of both L-glutamate and ATP, thus driving
the amino acid activation.

|

|
|
|

|
| |
Selected figure(s)
|
|

|
| |
 |
 |

|
 |

|
 |
Figure 3.
Figure 3. Glutamate Interaction Manners in the Absence and
Presence of tRNA^Glu
(A) A stereo view showing the manner
of glutamate recognition by the GluRS•tRNA^Glu complex in the
ERS/tRNA/Glu structure. The bound glutamate, the protein amino
acid residues, and A76 are colored green, white, and salmon,
respectively.
(B) The GluRS-glutamate interaction in the
ERS/Glu structure (stereo view). The glutamate molecule is
colored yellow.
|
 |
Figure 6.
Figure 6. Glutamol in ERS/tRNA/ATP/Eol and Glutamyl-Sulfamoyl
Adenosine in ERS/tRNA/ESA
(A) Schematic drawings of
L-glutamate (upper) and L-glutamol (Eol; lower) (Desjardins et
al., 1998).
(B) Schematic drawings of L-glutamyl-adenylate
(upper) and 5′-O-[N-(L-glutamyl)-sulfamoyl] adenosine (ESA;
lower). ESA is a nonhydrolyzable analog of glutamyl-adenylate,
and it is known as a potent competitive inhibitor of E. coli
GluRS with respect to glutamic acid (K[i] = 2.8 nM) (Bernier et
al., 2005).
(C) The annealed |F[o] − F[c]| omit electron
density contoured at 3 σ, showing the L-glutamol molecule in
ERS/tRNA/ATP/Eol. Two alternative conformations of the Eol
molecule (cyan and marine) are superimposed.
(D) The omit
electron density map corresponding to glutamyl-sulfamoyl
adenosine in ERS/tRNA/ESA. The refined ESA model (light green)
is superimposed on the density contoured at 3 σ.
|
 |
|
|

|
| |
The above figures are
reprinted
by permission from Cell Press:
Structure
(2006,
14,
1791-1799)
copyright 2006.
|
|
| |
Figures were
selected
by an automated process.
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.Palencia,
T.Crépin,
M.T.Vu,
T.L.Lincecum,
S.A.Martinis,
and
S.Cusack
(2012).
Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase.
|
| |
Nat Struct Mol Biol,
19,
677-684.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
T.Ito,
N.Kiyasu,
R.Matsunaga,
S.Takahashi,
and
S.Yokoyama
(2010).
Structure of nondiscriminating glutamyl-tRNA synthetase from Thermotoga maritima.
|
| |
Acta Crystallogr D Biol Crystallogr,
66,
813-820.
|
 |
|
PDB code:
|
 |
|
|
|
|
|
 |
E.M.Corigliano,
and
J.J.Perona
(2009).
Architectural underpinnings of the genetic code for glutamine.
|
| |
Biochemistry,
48,
676-687.
|
 |
|
|
|
|
 |
G.L.Igloi,
and
E.Schiefermayr
(2009).
Amino acid discrimination by arginyl-tRNA synthetases as revealed by an examination of natural specificity variants.
|
| |
FEBS J,
276,
1307-1318.
|
 |
|
|
|
|
 |
S.Paravisi,
G.Fumagalli,
M.Riva,
P.Morandi,
R.Morosi,
P.V.Konarev,
M.V.Petoukhov,
S.Bernier,
R.Chênevert,
D.I.Svergun,
B.Curti,
and
M.A.Vanoni
(2009).
Kinetic and mechanistic characterization of Mycobacterium tuberculosis glutamyl-tRNA synthetase and determination of its oligomeric structure in solution.
|
| |
FEBS J,
276,
1398-1417.
|
 |
|
|
|
|
 |
T.L.Bullock,
A.Rodríguez-Hernández,
E.M.Corigliano,
and
J.J.Perona
(2008).
A rationally engineered misacylating aminoacyl-tRNA synthetase.
|
| |
Proc Natl Acad Sci U S A,
105,
7428-7433.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
L.T.Guo,
X.L.Chen,
B.T.Zhao,
Y.Shi,
W.Li,
H.Xue,
and
Y.X.Jin
(2007).
Human tryptophanyl-tRNA synthetase is switched to a tRNA-dependent mode for tryptophan activation by mutations at V85 and I311.
|
| |
Nucleic Acids Res,
35,
5934-5943.
|
 |
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

