 |
PDBsum entry 2cib

|
|
|

|
 |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
 |

|

|

|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|

|

|

|

|

|

|
|
Oxidoreductase
|
PDB id
|
|

|

|
2cib
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|
 |
Contents |
 |
|
|

|

|
|
|
|
|
|
|
|
* Residue conservation analysis
|
|
|

|
|
PDB id:
|
 |
|
 |
| Name: |
 |
Oxidoreductase
|
 |
|
Title:
|
 |
High throughput screening and x-ray crystallography assisted evaluation of small molecule scaffolds for cyp51 inhibitors
|
|
Structure:
|
 |
Cytochrome p450 51. Chain: a. Synonym: sterol 14alpha-demethylase cyp51, ypli, p450-lia1, sterol 14-alpha demethylase, lanosterol 14-alpha demethylase, p450-14dm. Engineered: yes. Mutation: yes. Other_details: 4xhis tag at thE C-terminus
|
|
Source:
|
 |
Mycobacterium tuberculosis. Organism_taxid: 83332. Strain: h37rv. Expressed in: escherichia coli. Expression_system_taxid: 562.
|
|
Resolution:
|
 |
|
1.50Å
|
R-factor:
|
0.204
|
R-free:
|
0.226
|
|
|
Authors:
|
 |
L.M.Podust,Y.Kim,L.V.Yermalitskaya,J.P.Von Kries,M.R.Waterman
|
|
Key ref:
|
 |
L.M.Podust
et al.
(2007).
Small-molecule scaffolds for CYP51 inhibitors identified by high-throughput screening and defined by X-ray crystallography.
Antimicrob Agents Chemother,
51,
3915-3923.
PubMed id:
|
 |
|
Date:
|
 |
|
17-Mar-06
|
Release date:
|
03-Jul-07
|
|
|

|

|
|
PROCHECK
|

|

|
|
|
Headers
|
 |
|
|
References
|
|
|
|

|

|
|
P9WPP9
(CP51_MYCTU) -
Sterol 14alpha-demethylase from Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)
|
|

|

|
Seq:
Struc:
|
 |
 |
 |
451 a.a.
420 a.a.*
|

|

|

|

|
|

|

|

|
 |
 |

|
|
Key: |
 |
PfamA domain |
 |
 |
 |
Secondary structure |
 |
 |
CATH domain |
 |
|
*
PDB and UniProt seqs differ
at 2 residue positions (black
crosses)
|
|

|
|
|
 |
|
|
 |
 |
 |
 |
Enzyme class:
|
 |
E.C.1.14.15.36
- sterol 14alpha-demethylase (ferredoxin).
|
|
 |
 |
 |
 |
 |
Reaction:
|
 |
a 14alpha-methyl steroid + 6 reduced [2Fe-2S]-[ferredoxin] + 3 O2 + 5 H+ = a Delta14 steroid + formate + 6 oxidized [2Fe-2S]-[ferredoxin] + 4 H2O
|
 |
 |
 |
 |
 |
14alpha-methyl steroid
|
+
|
6
×
reduced [2Fe-2S]-[ferredoxin]
|
+
|
 3
×
O2
3
×
O2
|
+
|
5
×
H(+)
|
=
|
Delta(14) steroid
|
+
|
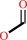 formate
formate
|
+
|
6
×
oxidized [2Fe-2S]-[ferredoxin]
|
+
|
4
×
H2O
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Molecule diagrams generated from .mol files obtained from the
KEGG ftp site
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|

|

|
| |
|
|
| |
|
|
Antimicrob Agents Chemother
51:3915-3923
(2007)
|
|
PubMed id:
|
|
|
|

|
| |
|
Small-molecule scaffolds for CYP51 inhibitors identified by high-throughput screening and defined by X-ray crystallography.
|
|
L.M.Podust,
J.P.von Kries,
A.N.Eddine,
Y.Kim,
L.V.Yermalitskaya,
R.Kuehne,
H.Ouellet,
T.Warrier,
M.Alteköster,
J.S.Lee,
J.Rademann,
H.Oschkinat,
S.H.Kaufmann,
M.R.Waterman.
|
|
|

|
| |
ABSTRACT
|
|

|
| |

|
Sterol 14alpha-demethylase (CYP51), a major checkpoint in membrane sterol
biosynthesis, is a key target for fungal antibiotic therapy. We sought small
organic molecules for lead candidate CYP51 inhibitors. The changes in CYP51
spectral properties following ligand binding make CYP51 a convenient target for
high-throughput screening technologies. These changes are characteristic of
either substrate binding (type I) or inhibitor binding (type II) in the active
site. We screened a library of 20,000 organic molecules against Mycobacterium
tuberculosis CYP51 (CYP51(Mt)), examined the top type I and type II binding hits
for their inhibitory effects on M. tuberculosis in broth culture, and analyzed
them spectrally for their ability to discriminate between CYP51(Mt) and two
reference M. tuberculosis CYP proteins, CYP130 and CYP125. We determined the
binding mode for one of the top type II hits,
alpha-ethyl-N-4-pyridinyl-benzeneacetamide (EPBA), by solving the X-ray
structure of the CYP51(Mt)-EPBA complex to a resolution of 1.53 A. EPBA binds
coordinately to the heme iron in the CYP51(Mt) active site through a lone pair
of nitrogen electrons and also through hydrogen bonds with residues H259 and
Y76, which are invariable in the CYP51 family, and hydrophobic interactions in a
phylum- and/or substrate-specific cavity of CYP51. We also identified a second
compound with structural and binding properties similar to those of EPBA,
2-(benzo[d]-2,1,3-thiadiazole-4-sulfonyl)-2-amino-2-phenyl-N-(pyridinyl-4)-acetamide
(BSPPA). The congruence between the geometries of EPBA and BSPPA and the CYP51
binding site singles out EPBA and BSPPA as lead candidate CYP51 inhibitors with
optimization potential for efficient discrimination between host and pathogen
enzymes.

|

|
|
|

|

|
|
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
Literature references that cite this PDB file's key reference
|
|
 |
| |
PubMed id
|
 |
Reference
|
 |
|
|
|
 |
A.G.Warrilow,
C.M.Martel,
J.E.Parker,
N.Melo,
D.C.Lamb,
W.D.Nes,
D.E.Kelly,
and
S.L.Kelly
(2010).
Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51).
|
| |
Antimicrob Agents Chemother,
54,
4235-4245.
|
 |
|
|
|
|
 |
C.Sheng,
S.Chen,
H.Ji,
G.Dong,
X.Che,
W.Wang,
Z.Miao,
J.Yao,
J.Lü,
W.Guo,
and
W.Zhang
(2010).
Evolutionary trace analysis of CYP51 family: implication for site-directed mutagenesis and novel antifungal drug design.
|
| |
J Mol Model,
16,
279-284.
|
 |
|
|
|
|
 |
H.Ouellet,
J.B.Johnston,
and
P.R.Ortiz de Montellano
(2010).
The Mycobacterium tuberculosis cytochrome P450 system.
|
| |
Arch Biochem Biophys,
493,
82-95.
|
 |
|
|
|
|
 |
P.S.Doyle,
C.K.Chen,
J.B.Johnston,
S.D.Hopkins,
S.S.Leung,
M.P.Jacobson,
J.C.Engel,
J.H.McKerrow,
and
L.M.Podust
(2010).
A nonazole CYP51 inhibitor cures Chagas' disease in a mouse model of acute infection.
|
| |
Antimicrob Agents Chemother,
54,
2480-2488.
|
 |
|
|
|
|
 |
T.C.Pochapsky,
S.Kazanis,
and
M.Dang
(2010).
Conformational plasticity and structure/function relationships in cytochromes P450.
|
| |
Antioxid Redox Signal,
13,
1273-1296.
|
 |
|
|
|
|
 |
C.K.Chen,
P.S.Doyle,
L.V.Yermalitskaya,
Z.B.Mackey,
K.K.Ang,
J.H.McKerrow,
and
L.M.Podust
(2009).
Trypanosoma cruzi CYP51 Inhibitor Derived from a Mycobacterium tuberculosis Screen Hit.
|
| |
PLoS Negl Trop Dis,
3,
e372.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
C.Sheng,
Z.Miao,
H.Ji,
J.Yao,
W.Wang,
X.Che,
G.Dong,
J.Lü,
W.Guo,
and
W.Zhang
(2009).
Three-dimensional model of lanosterol 14 alpha-demethylase from Cryptococcus neoformans: active-site characterization and insights into azole binding.
|
| |
Antimicrob Agents Chemother,
53,
3487-3495.
|
 |
|
|
|
|
 |
L.M.Podust,
H.Ouellet,
J.P.von Kries,
and
P.R.de Montellano
(2009).
Interaction of Mycobacterium tuberculosis CYP130 with heterocyclic arylamines.
|
| |
J Biol Chem,
284,
25211-25219.
|
 |
|
PDB codes:
|
 |
|
|
|
|
|
 |
A.N.Eddine,
J.P.von Kries,
M.V.Podust,
T.Warrier,
S.H.Kaufmann,
and
L.M.Podust
(2008).
X-ray structure of 4,4'-dihydroxybenzophenone mimicking sterol substrate in the active site of sterol 14alpha-demethylase (CYP51).
|
| |
J Biol Chem,
283,
15152-15159.
|
 |
|
PDB code:
|
 |
|
|
 |
 |
|
The most recent references are shown first.
Citation data come partly from CiteXplore and partly
from an automated harvesting procedure. Note that this is likely to be
only a partial list as not all journals are covered by
either method. However, we are continually building up the citation data
so more and more references will be included with time.
Where a reference describes a PDB structure, the PDB
codes are
shown on the right.
|
 |

